Figure 6.
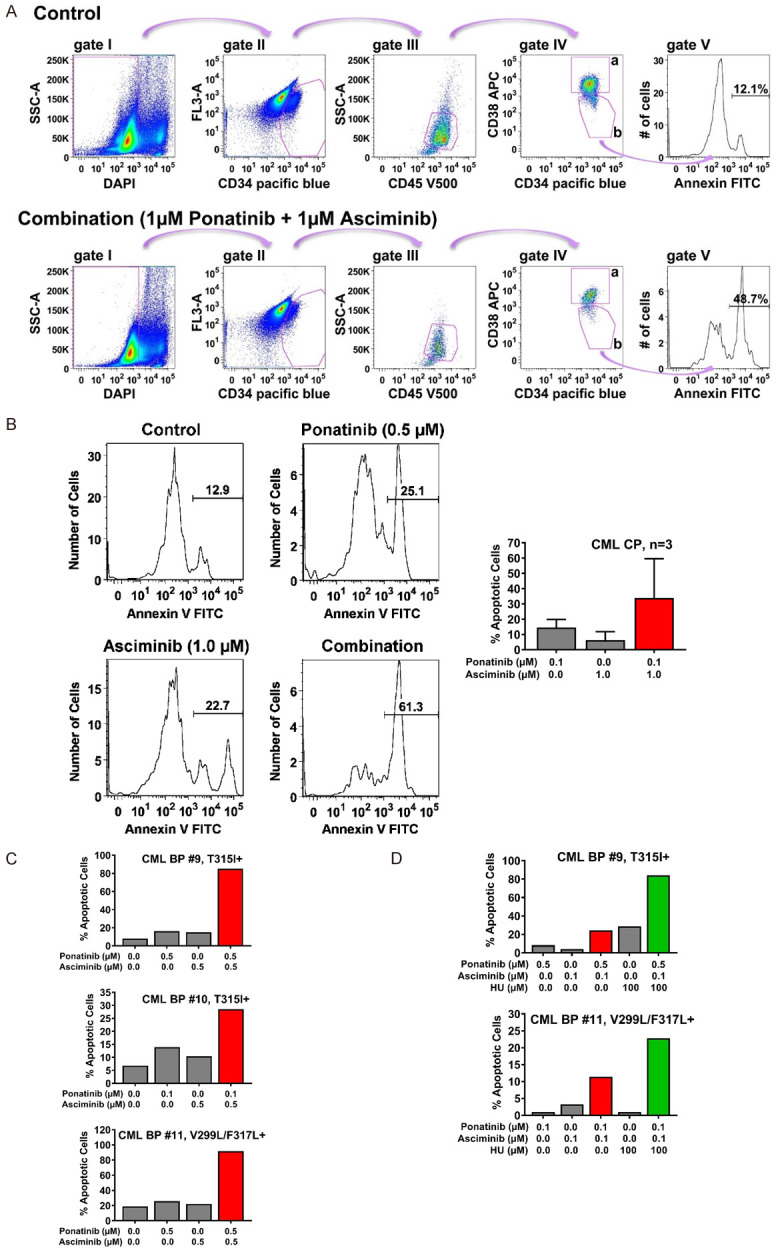
Asciminib, ponatinib and HU synergize in inducing apoptosis in CD34+/CD38- CML stem cells. A. For the detection of Annexin+/DAPI- immature CD34+/CD45dim/CD38- stem cells in peripheral blood samples of CML patients, a step by step gating strategy was applied (steps I-IV) as indicated by pink arrows. After excluding DAPI-negative cells (gate I), immature cells were identified based on their CD34 expression (gate II) and further gated as CD45 dim-positive cells (gate III). Next, cells were separated into progenitor cells (CD34+/CD38+; gate IVa) and stem cells (CD34+/CD38-; gate IVb) by their CD38 expression. Expression of Annexin was determined in stem cells as shown in gate V. The example shows peripheral blood cells from a patient with CML treated with control medium (upper panel) or the combination of ponatinib and asciminib (each 1 µM, lower panel) for 48 hours. B. Primary neoplastic cells isolated from 3 patients with CP CML (patients #1, #2 and #3) were kept in control medium or in the presence of asciminib, ponatinib, or a combination of both drugs as indicated for 48 hours before apoptosis in CD34+/CD38- stem cells was determined by Annexin V-FITC/DAPI staining and flow cytometry. One typical experiment (patient #1) is shown in the left panel; results shown in the right panel represent the mean ± S.D. of the percentage of apoptotic cells (after subtraction of apoptotic cell-counts in control medium) in each condition determined in three patients (patients #1, #2 and #3). C, D. Primary neoplastic cells isolated from 3 patients with BP CML (patients #9, #10 and #11) were kept in control medium or in the presence of asciminib, ponatinib, HU or drug-combinations as indicated for 48 hours before apoptosis within the CD34+/CD38- stem-cell fraction was determined by Annexin V-FITC/DAPI staining and flow cytometry. Results show one typical experiment after subtraction of apoptotic cell-counts in control medium. Patients’ numbers refer to Table 1.
