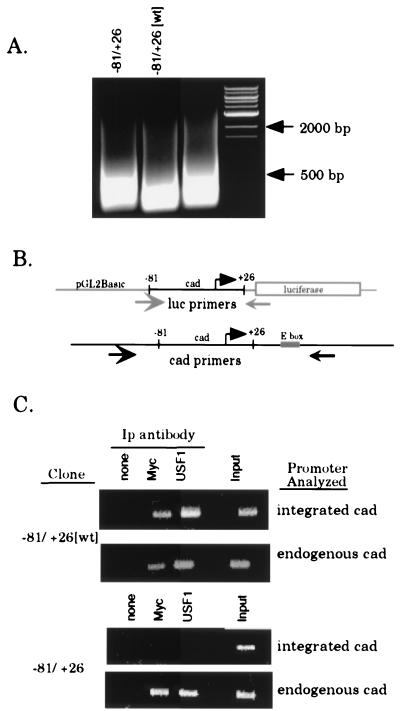
FIG. 5.
Myc and USF1 bind specifically to the E box within the cad promoter. Clones of stably transfected cells (−81/+26[wt] clone 1 and −81/+26 clone 5) were serum starved for 48 h, then serum stimulated for 8 h, and cross-linked with formaldehyde. Cross-linked chromatin from each line was prepared and immunoprecipitated with antibodies against Myc and USF1. (A) EtBr-stained agarose gel showing the size of the DNA fragments following sonication. Lanes were loaded with 2.5% input chromatin from the specified clone after reversal of the cross-links and proteinase K treatment. DNA size markers are as indicated. (B) Schematic of the annealing position of PCR primers used to specifically amplify either integrated or endogenous cad promoters. The luciferase and cad primers amplify products of 200 and 350 bp, respectively. (C) PCR analysis of immunoprecipitation reactions with luciferase or cad primers to amplify the integrated or endogenous cad promoter, respectively. For all stable clones, immunoprecipitates were resuspended in 30 μl of H2O and input chromatin samples were diluted to 1% in 100 μl of H2O. The copy number of the integrated plasmid was normalized by utilizing appropriate sample volumes such that the input chromatin signal for all clones analyzed was approximately equivalent (last lane of each upper panel, integrated cad). Volumes used for both input chromatin and the immunoprecipitates (Ip) were as follows: 3 μl of the −81/+26-5 samples and 2 μl of −81/+26[wt]-1 samples. PCR mixtures with the cad primers (endogenous cad) contained equivalent sample volumes (2 μl) for all clones examined. PCR products were electrophoresed on a 1.5% agarose gel and stained with EtBr.