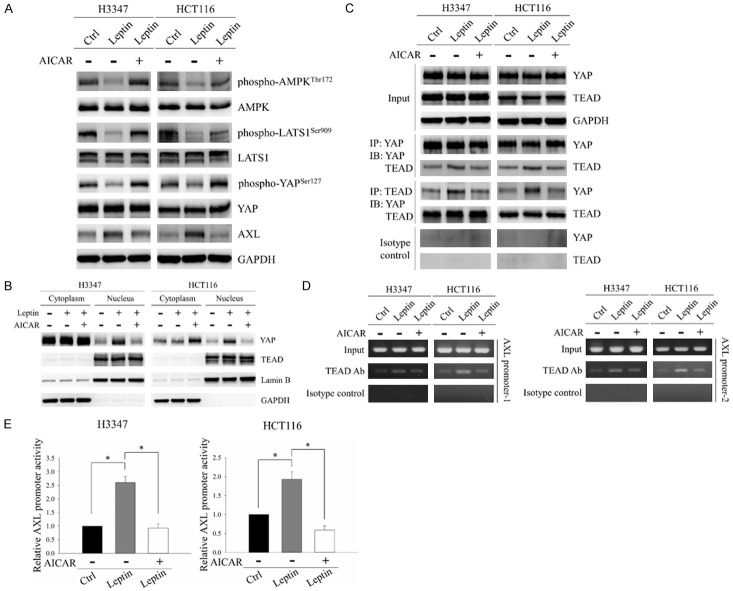
Figure 4.
AMPK was the downstream regulator of leptin-induced activation of the YAP-TEAD complex in CRC cells. H3347 and HCT116 cells were treated with 100 ng/ml leptin recombinant protein with/without 0.5 mM of the AMPK activator, AICAR, for 48 hours. A. Protein levels of phospho-AMPK (Thr172), AMPK, phospho-LATS1 (Ser909), LATS1, phospho-YAP (Ser127), YAP and AXL were analyzed by Western blot analysis. B. Subcellular distributions of YAP and TEAD were investigated by Western blot analysis of the cytoplasmic and nuclear fractions of CRC cells after the indicated treatments. Lamin B and GAPDH served as nuclear and cytoplasmic controls, respectively. C. The interaction between YAP and TEAD was evaluated using co-immunoprecipitation. Protein extracts of CRC cells were immunoprecipitated with anti-YAP or anti-TEAD antibodies. The expressions of YAP and TEAD in the precipitated proteins were detected by Western blot analysis. D. The association between TEAD and the AXL promoter was examined by ChIP assay. The ChIP assay was carried out on the AXL promoter using anti-TEAD antibodies followed by PCR to amplify the DNA region containing TEAD binding sites. E. pAXL-cypridina luc plasmid and internal control pTK-red firefly luc plasmid were co-transfected into H3347 and HCT116 cells followed by treatment with 100 ng/ml leptin recombinant protein with/without 0.5 mM AICAR for 48 hours. The relative promoter activity of AXL was subsequently detected using a dual luciferase assay. GAPDH served as the loading control. Anti-rabbit IgG was used as the isotype control. Data are expressed as the mean ± SEM. SEM, error bars. *P<0.05 by one-way ANOVA followed by Bonferroni’s post hoc test.