Figure 1. . Synthesis of HA-PEI/PEG-M2 peptide nanoparticles.
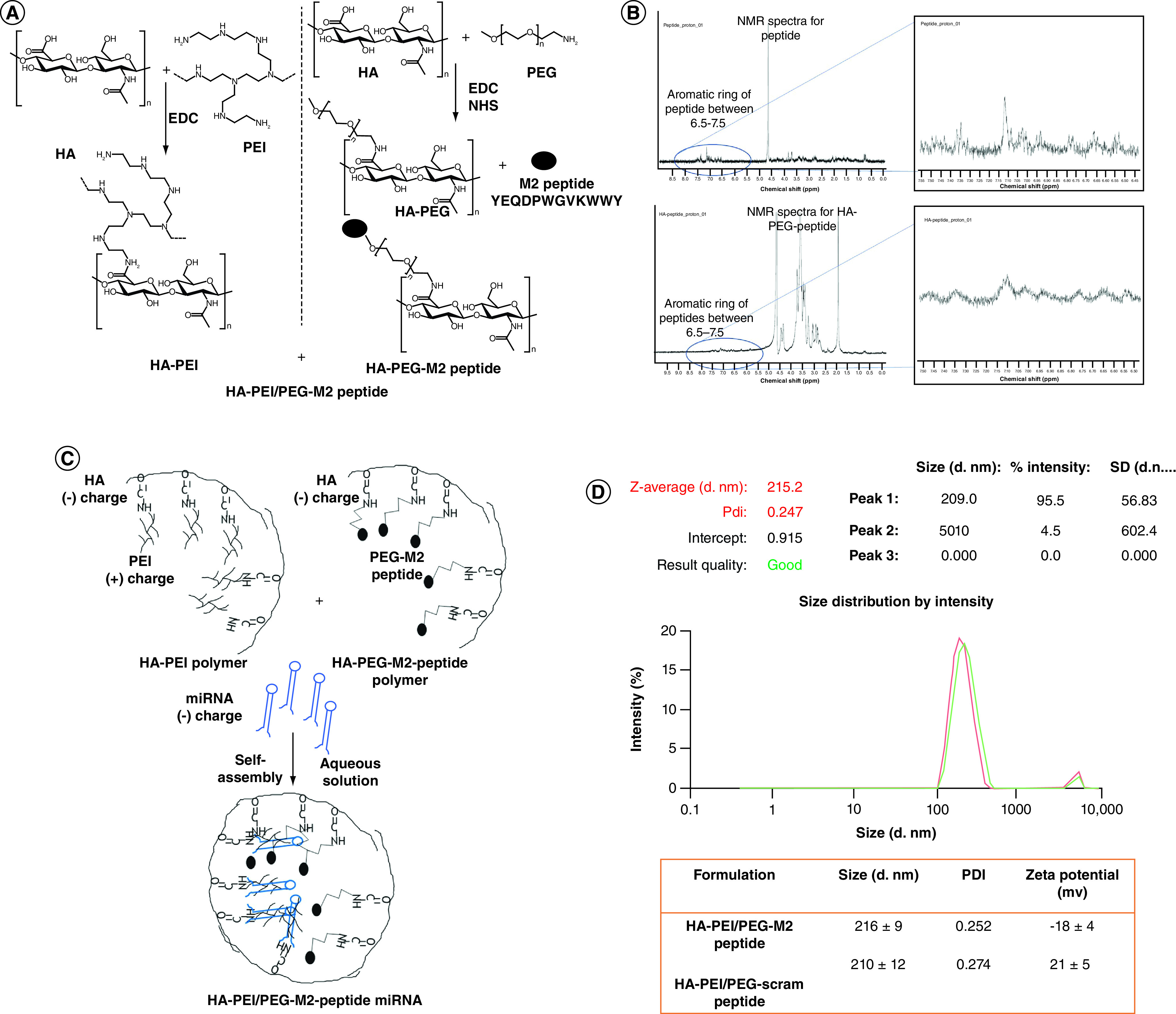
(A) Synthesis and cancerization of HA-PEI, HA-PEG and HA-PEI/HA/PEG-peptide conjugates. HA-PEI and HA-PEG-maleimide derivatives were synthesized using a simple and versatile EDC/NHS conjugation chemistry. M2 peptide was further conjugated to HA-PEG-maleimide. Both the HA polymers are used in a 1:1 ratio for encapsulation of miRNA-125b. (B) The 1H-NMR spectra of the peptide show the characteristic aromatic ring peaks between 6.5–7.5 p.p.m. The 1H-NMR spectra of the HA-PEG-peptide also shows the characteristic aromatic ring peaks between 6.5–7.5 ppm. (C) A-PEI/PEG M2 peptide miRNA nanoparticle formation (D) Hydrodynamic diameters, PDI and ζ-potentials of HA-PEI/HA-PEG-Scram peptide or HA-PEI/HA-PEG-M2 peptide nanoparticle formulations. n = 4 identically prepared samples; data are expressed as mean ± SD.
EDC: Ethyl(dimethylaminopropyl) carbodiimide; HA: Hyaluronic acid; HA-PEG: Hyaluronic acid-polyethylene glycol; HA-PEI: Hyaluronic acid-polyethyleneimine; M2: Alternatively activated macrophage; NHS: N-hydroxysuccinimide; NMR: Nuclear magnetic resonance; PDI: Polydispersity index; PEG: Poly(ethylene glycol); PEI: Polyethyleneimine; SD: Standard deviation.
