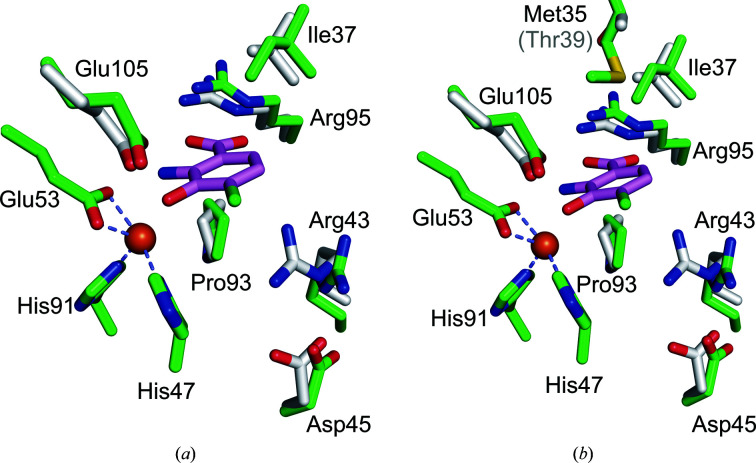
Figure 8.
Superposition of key active-site residues of Fe(III)-h3HAO (green C atoms) and R. metallodurans 3HAO (gray C atoms) in complex with the inhibitor 4-Cl-3-HANA (magenta C atoms). Fe atoms are shown as orange spheres. (a) The active sites superimposed showing that all of the residues implicated in inhibitor binding are structurally well conserved, with the exception that Arg47 in R. metallodurans 3HAO, which interacts with bound oxygen in the complex (not shown), shifts relative to its Fe(III)-h3HAO counterpart Arg43. (b) The same view but with the nonconserved Met35 shown in comparison to its R. metallodurans 3HAO counterpart Thr39. Met35 projects into the active site near, but not close enough to interact with, a bound ligand. However, a minor reorientation of Met35 could result in a van der Waals interaction with a substrate or inhibitor.