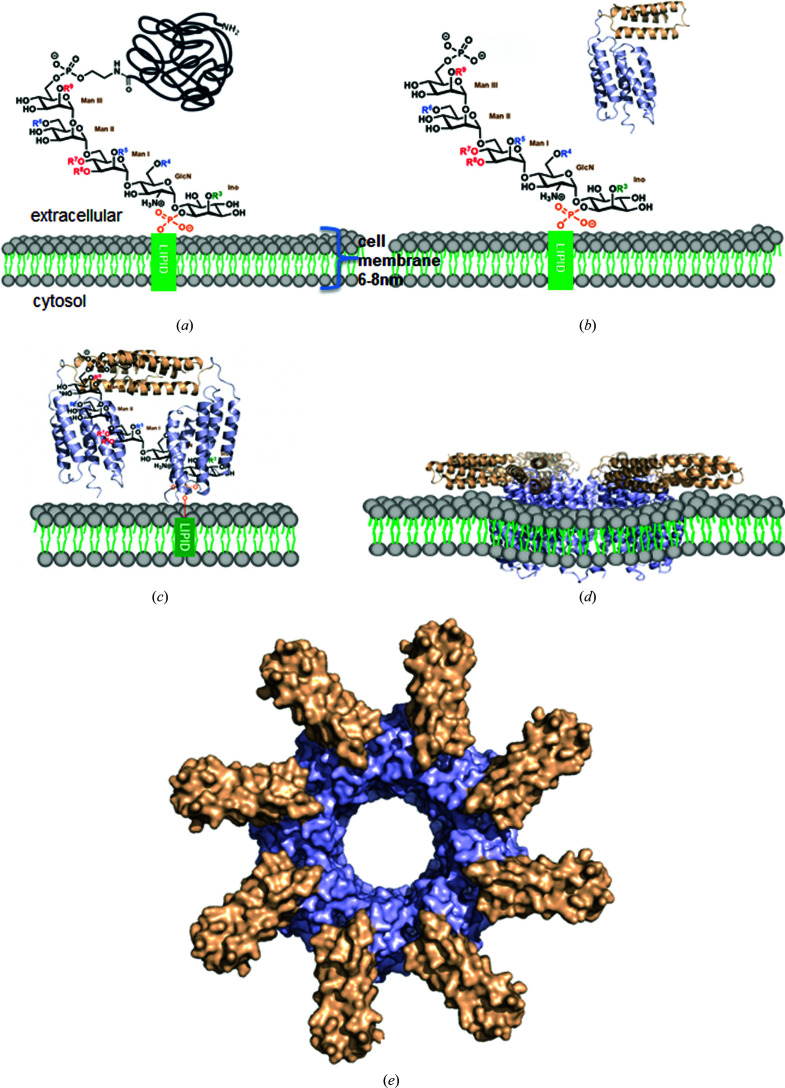
Figure 6.
Proposed working model for CAMP factor. (a) Chemical structure of a GPI anchor on the cell surface. (b) The action of sphingomyelinase cleaves neutrally charged phosphatidylcholine in the cell membrane, which exposes the negatively charged outer surface of the cell membrane and facilitates the binding of monomeric soluble CAMP factors to the negatively charged phosphate-sugar moieties of GPI. (c) Attachment to the cell surface through binding of the CTD to the GPI anchor. The positively charged patches on the H6–H7 surface of the CTD bind to phosphate-sugar moieties of the GPI anchor, which leads to the dimerization of CAMP-factor molecules through the CTD along with bound GPI anchors. The NTD is oriented towards the membrane, suitable for membrane insertion. (d) Membrane insertion of the NTD and structural rearrangement in a lipid bilayer with unknown mechanism. (e) A hypothetical model of the membrane-inserted pore. The model was generated by symmetric docking of eight CAMP-factor molecules through NTD interactions.