Abstract
Visual agnosia is a neuropsychological impairment of visual object recognition despite near-normal acuity and visual fields. A century of research has provided only a rudimentary account of the functional damage underlying this deficit. We find that the object-recognition ability of agnosic patients viewing an object directly is like that of normally-sighted observers viewing it indirectly, with peripheral vision. Thus, agnosic vision is like peripheral vision. We obtained 14 visual-object-recognition tests that are commonly used for diagnosis of visual agnosia. Our “standard” normal observer took these tests at various eccentricities in his periphery. Analyzing the published data of 32 apperceptive agnosia patients and a group of 14 Posterior Cortical Atrophy (PCA) patients on these tests, we find that each patient’s pattern of object recognition deficits is well characterized by one number, the equivalent eccentricity at which our standard observer’s peripheral vision is like the central vision of the agnosic patient. In other words, each agnosic patient’s equivalent eccentricity is conserved across tests. Across patients, equivalent eccentricity ranges from 4 to 40 deg, which rates severity of the visual deficit.
In normal peripheral vision, the required size to perceive a simple image (e.g. an isolated letter) is limited by acuity, and that for a complex image (e.g. a face or a word) is limited by crowding. In crowding, adjacent simple objects appear unrecognizably jumbled unless their spacing exceeds the crowding distance, which grows linearly with eccentricity. Besides conservation of equivalent eccentricity across object-recognition tests, we also find conservation, from eccentricity to agnosia, of the relative susceptibility of recognition of ten visual tests. These findings show that agnosic vision is like eccentric vision.
Whence crowding? Peripheral vision, strabismic amblyopia, and possibly apperceptive agnosia are all limited by crowding, making it urgent to know what drives crowding. Acuity does not (Song et al., 2014), but neural density might: neurons per deg2 in the crowding-relevant cortical area.
1. INTRODUCTION
Visual apperceptive agnosia
Visual agnosia is a neuropsychological disorder characterized by the inability to recognize familiar objects. Visual agnosia patients are generally unable to recognize visually presented objects, but they can successfully name the object on the basis of tactile exploration and they correctly describe the object’s function from its name. Such impairment must be distinguished from early sensory deficits (e.g. low visual acuity or contrast sensitivity), oculomotor disturbances, attentional deficits, aphasic syndromes, and mental deterioration (Farah, 1990; De Renzi, 1996). It is remarkable that these patients can recognize a tiny letter when tested for acuity, yet cannot recognize everyday objects.
The nature of visual agnosia is debated, and patients within this gross category are diverse. For a recent review, see Behrmann & Nishimura (2010). Neuropsychological studies of brain-damaged patients have found selective deficits for words (pure alexia), objects (pure visual object agnosia), and faces (prosopagnosia) (Farah 2004). The inhomogeneity of the visual agnosic population reported in the literature may reflect the various neural sites of the lesion and the varying degree of neural damage (Adler, 1944; Benson and Greenberg, 1969; Campion and Latto, 1985; Milner et al., 1991; Vecera and Behrmann, 1997; Behrmann & Nishimura, 2010). The classical description (Lissauer, 1890/1988) distinguishes “apperceptive” agnosia, which is a perceptual processing deficit, from “associative” agnosia, which is a deficit either in semantic knowledge of visual objects or in accessing that knowledge. Associative agnosia patients have trouble recognizing a variety of visually presented objects, despite their intact visual perception, which is usually demonstrated by having the patients copy objects that they cannot recognize. Patients that show highly selective impairment of one object category (i.e., pure alexia, pure visual object agnosia, and prosopagnosia) are classically said to have an associative deficit (Farah, 2004). We will consider the debate about category-specific deficits in visual agnosia in the Discussion section. Putting associative deficits aside, here we focus on apperceptive agnosia. Loosely, “apperceptive” refers to sensation without perception, marked by detection without recognition. The apperceptive deficit comprises a broad range of symptoms. Several authors have proposed a detailed taxonomy of visual apperceptive agnosia, differentiating it into: shape/form agnosia (Efron, 1968; Milner et al., 1991; Riddoch et al., 2008), integrative agnosia (Riddoch & Humphreys, 1987), transformational agnosia (Warrington, 1985; Humphreys & Riddoch, 1987), and perceptual categorization deficit (Farah, 2004). The deficits range from severe — in patients who cannot even discriminate simple geometric shapes (shape agnosia) — to mild — in patients who seem unimpaired in their daily lives, but who fail, at the clinic, to recognize familiar objects in photographs taken from unusual perspectives (transformational agnosia). Between these two extremes, there is a wide intermediate range of deficit that is sometimes called “integrative agnosia” (Riddoch and Humphreys, 1987). Here, we apply the term apperceptive agnosia to this broad category of patients with an intermediate degree of deficit. These patients with visual agnosia are profoundly impaired in object recognition, face recognition, word recognition, and reading. They may show signs of achromatopsia and topographical agnosia as well. They do recognize an isolated letter. They typically perform better with real objects than with drawings and photographs, but only if the objects are presented in isolation or in motion. This syndrome is usually associated with either bilateral occipito-temporal lesions or unilateral right occipito-temporal lesion sparing striate cortex and parietal areas (Humphreys, 1999).
Popularized by Oliver Sacks (1998) in “The man who mistook his wife for a hat,” apperceptive agnosia has long attracted keen interest for the investigation of integration in object recognition and how we produce a single coherent percept (Lissauer, 1890; Riddoch and Humphreys, 1987; Behrmann and Kimchi, 2003). Despite severely impaired visual recognition of the object, these patients can verbally describe what they perceive, though their descriptions are often piecemeal. When presented with a drawing of a paintbrush, HJA (one of the most famous and well-studied cases of visual agnosia) said, “it appears to be two things close together; a longish wooden stick and a shorter, darker object, though this can’t be right or you would have told me.” (Riddoch and Humphreys, 1987, p. 60).
Despite detailed descriptions of individual patients spanning the whole range of symptoms associated with this syndrome, there is still no comprehensive account. According to Riddoch and Humphreys (1987), apperceptive agnosia is an “integration deficit”: The patients can process local visual elements but cannot integrate them into a whole. However, contrary to this generalization, some apperceptive agnosia patients perform better with silhouettes than with drawings (Humphreys, Riddoch and Quinlan, 1985; Lê et al., 2002), which presumably requires some integration (Humphreys, 1999). On the other hand, these patients are still impaired in recognizing a single part of a complex object. For instance, they are slower than normally-sighted observers in processing a “local” letter embedded in “global” letter (Navon, 1977; Behrmann and Kimchi, 2003). The interplay between impairments of recognition of single parts and complex objects remains mysterious.
Apperceptive agnosia severely impairs vision yet spares acuity and visual fields. Patients with visual agnosia can recognize small simple shapes (e.g. a letter) when presented in isolation. Most visual impairments (e.g. macular degeneration or anisometropic amblyopia) restrict visual field or acuity, and are well characterized by those restrictions. However, there are several conditions, like apperceptive agnosia, that impair central vision while sparing acuity and fields. We focus on perceptual deficits (hence apperceptive agnosia), putting aside high-level attentional deficits such as neglect and simultanagnosia (and associative agnosias). The perceptual deficits of central vision that spare acuity include: apperceptive agnosia, achromatopsia (color agnosia), akinetopsia (motion blindness), dysmetropsia (failure of size constancy), transformational agnosia (inability to recognize objects seen from an unusual perspective), and depth perception deficits. Among them, only apperceptive agnosia specifically impairs recognition of complex shapes.
Here, we provide evidence towards a simple unified account of apperceptive agnosia. We show that apperceptive agnosia is like peripheral vision, which is limited by visual crowding.
Visual crowding
Visual crowding is the failure to identify a simple object (like a letter) because of surrounding clutter. When the clutter closely surrounds the target object, the features of the target and clutter mingle together, producing a jumble that is hard to identify. Recognition is wrecked, but detection is unscathed. This perceptual phenomenon was reported first in the foveal vision of amblyopes, and then in normal peripheral vision (Korte, 1923; Irvine, 1945). It was later dubbed “crowding” (Ehlers,1953; Stuart and Burian, 1962). In strabismic amblyopia, the acuity size for identifying a foveal letter is raised ten-fold when other letters surround the target (Levi, Song, & Pelli, 2007). In the normal fovea, crowding is usually negligible, occurring only when clutter is within a few minutes of arc (Flom, Heath, & Takahaski, 1963; Latham, & Whitaker, 1996; Liu & Arditi, 2000; Pelli et al., 2016). Crowding severely limits peripheral vision (Levi et al., 2007; Song, Levi & Pelli, 2014).
Crowding is usually characterized by its extent, the crowding distance (or “critical spacing”), defined as the minimum distance, center-to-center, between a simple target and a neighboring clutter object, beyond which the clutter is innocuous. Crowding distance grows linearly with eccentricity (angular distance from the point of fixation). This “Bouma law” holds for most objects and tasks (Bouma 1970; Toet and Levi, 1992; Martelli et al., 2005; Pelli & Tillman, 2008; Pelli et al., 2007; Pelli et al., 2004; Whitney & Levi, 2011), though crowding distance may be reduced somewhat through familiarity (Grainger et al., 2010; Chung, 2007). Thus, as eccentricity is increased from zero (at fixation) to 60 degrees, crowding distance increases from 0.05 to 18 degrees, nearly 400:1 (Eq. 2). The wide range of crowding in the normal periphery has allowed extensive study. (For reviews, see Whitney & Levi, 2011; Levi, 2008; Pelli & Tillman, 2008; Pelli, Palomares, & Majaj, 2004.)
The crowding distance distinguishes crowding from ordinary “overlap” masking. In overlap masking, the flanker and target overlap (or are nearly contiguous), and the flanker-to-target center-to-center spacing needed for identification depends on stimulus size and not on eccentricity, whereas the crowding distance depends on stimulus eccentricity and not on size (Pelli et al., 2004). Overlap masking makes the target unrecognizable and invisible, presumably because the detector in the primary visual cortex also responds to the flankers (Pelli et al., 2004; Thomas, 1985; Legge & Foley, 1980). The target is also unrecognizable in crowding (and agnosia), but remains visible. Crowding combines detected features over an inappropriately large area, producing a jumbled percept. Models of crowding suppose pooling or source confusion (Levi, 2008; Nandy & Tjan, 2007; Chung et al., 2007; Martelli, Majaj, & Pelli, 2005; Parkes et al., 2001; Treisman & Gelade, 1980).
Crowding is typically manifest in one of two ways, depending on the complexity of the target. A simple target, like a Roman letter A-Z, is only crowded if other objects are nearby, within the crowding distance. Alternatively, in a complex target with several parts, like a word, the parts (letters) can crowd each other (Rosen et al., 2014). This is self-crowding. The crowding distance of the elements (e.g. letters) is the same. There is not yet an independent definition for what constitutes a “part”, other than the self-crowding test. But, so far, all parts have turned out to have familiar names. Thus, Martelli et al. (2005) found that a face is complex, like a word, and consists of facial features — eyes, nose, and mouth — that must be at least the crowding distance apart for the face to be recognized. Consequently, in the periphery, a shrunken target remains identifiable only if it is simple, like a letter or a facial feature, while a complex target, like a word or a face, can only be identified if it is huge, large enough so that its parts do not crowd each other. Thus, for an isolated object in the periphery, the minimum size required for identification is determined by visual acuity if it is simple, and by crowding if it is complex (Pelli et al., 2004). In sum, the crowding phenomenon is a severe and distinctive impairment of recognition.
Two research groups have reported crowding in neuropsychological patients. Price and Humphreys (1995) reported that two alexic patients identified letters in a string more accurately when they were widely spaced, and this effect was most pronounced for the central letters in the string. Using Bouma’s (1970) terminology, they called this crowding effect an abnormally strong “flanker interference” in letter identification. Crutch and Warrington (2007; 2009) found that the reading deficits of two patients with bilateral posterior cortical atrophy (PCA) could be attributed to crowding: Letter identification accuracy decreased in the presence of flankers, more so with greater flanker proximity, independent of target or flanker size. After our initial submission of this manuscript, Crutch and co-authors reported crowding tests and brain imaging for 26 PCA patients (Yong, Shakespeare, Cash, Henley, Nicholas, Ridgway, Golden, Warrington, Carton, Kaski, Schott, Warren, Crutch, 2014). Again, they found crowding: The PCA patients were less accurate and slower to identify targets between flankers when the flankers were nearer, and this effect was correlated with lower grey matter volume. The two Crutch and Warrington patients are included in our sample, but the Yong et al. (2014) paper was published too late to be included.
The crowding conjecture for apperceptive agnosia
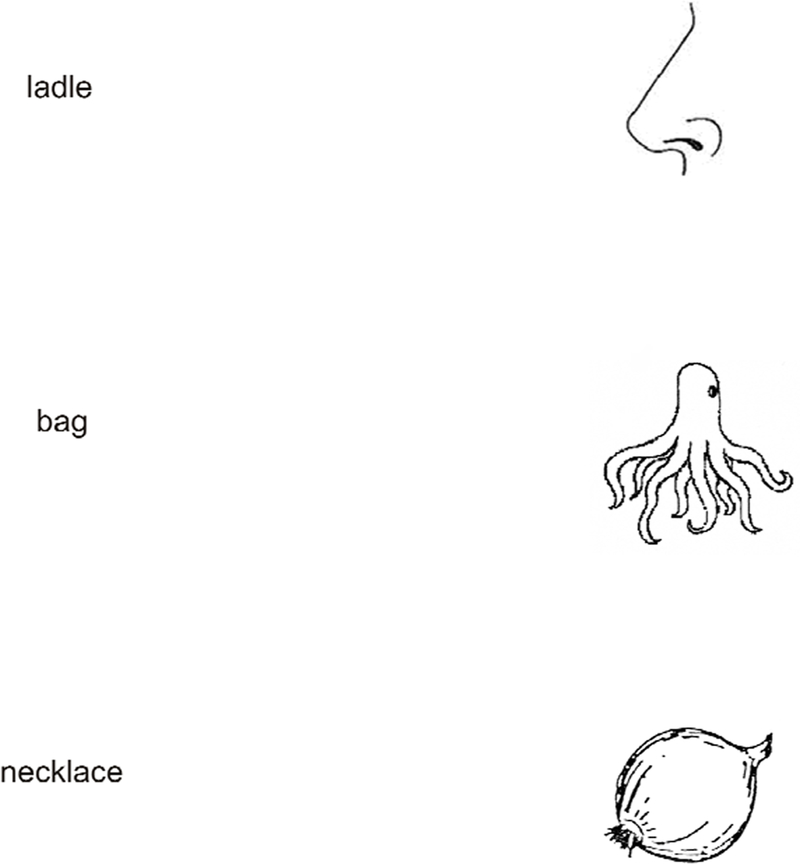
Crowding is an important well-studied operationally-defined psychophysical phenomenon. Establishing that a visual impairment is “crowding” classifies it as this well-known perceptual phenomenon. Here we link apperceptive agnosia and crowding, and we anticipate that this link will prove useful in elucidating both. Specifically, we conjecture that the deficit in agnosic central vision is like the crowding at some eccentricity in normal peripheral vision. It predicts that the agnosic patient sees a simple display, e.g. an isolated letter, normally, but has impaired vision of complex displays, like a normally-sighted observer viewing peripherally (Fig. 1), and recognition is limited by the spacing of the simple objects that make up the complex display. Towards our goal of showing the similarity of agnosic and peripheral vision, we begin by allowing our readers to compare their peripheral impressions with verbal reports from agnosic patients. We hope this will make our proposal clear, and set the stage for the formal tests described in Methods and Results.
Figure 1. What they say.
Compare central agnosic vision to your own peripheral vision. The drawings on the right (Snodgrass and Vanderwart, 1980) are often presented to patients to test for visual agnosia. Each drawing appears to the right of the word response that it elicited from an agnosic patient (“ladle” and “necklace” from HJA in Humphreys and Riddoch, 1987, and “bag” from SM in Behrman and Kimchi, 2003). To experience something like agnosic vision, please fixate each word, and, without moving your eyes, try to identify the object in your right peripheral field. You may find yourself agreeing with the patients.
A hint of this idea emerges in Humphreys & Riddoch’s (1987, p. 78) description of the perceptual world of HJA as, “composed of rather gross descriptions of objects — the kinds of descriptions we might make if we glimpsed objects from the corner of an eye.” Moreover, in crowding, a normally-sighted observer can report something of what he sees peripherally, even if he cannot identify it. In crowding, the target is visible but jumbled. In line with this phenomenology, the agnosia patient FWT said that everything seemed to “run together” (Shelton, Bowers, Duara & Heilman, 1994). Gordon (1968) describes the recognition abilities of a child with early-acquired visual agnosia, “although he can recognize pictures of objects if presented singly, he cannot always identify the same objects if several pictures are presented on the same card.” Similarly, HJA reported that, in ordinary life, he found it much harder to recognize objects close to each other: “For instance, eating at a buffet or a self-service restaurant is extremely difficult. I can recognize many food items seen individually. They somehow seem hard to separate en masse” (Humphreys & Riddoch, 1987, p. 33).
Figure 1 allows you to compare your perceptual experience of peripherally seen objects with the patients’ descriptions. While looking directly at the object, the patient’s mistaken name is absurd, but the same name seems appropriate when the object is viewed peripherally while fixating the name. We conjecture that agnosic vision is like peripheral vision, and thus that the agnosic deficit is like crowding.
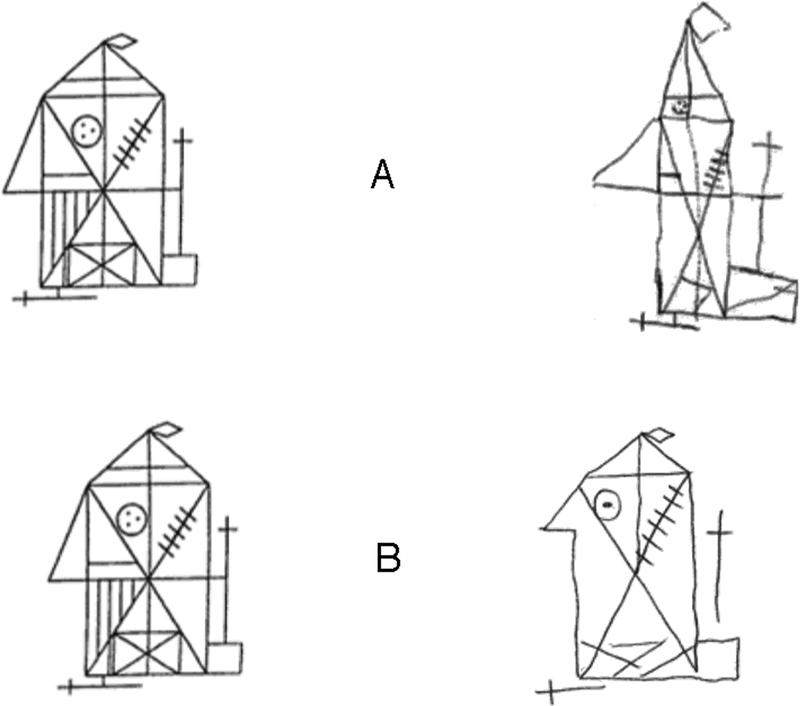
Copying a drawing has long been important in the neuropsychological assessment of visual agnosia. Figure 2 shows the standard Rey complex figure on the left and a copy on the right, made by an apperceptive agnosia patient (Rey, 1941; Lê et al. 2002). The patient was simply asked to copy, with unrestricted viewing. As a preliminary test of our conjecture that agnosic central vision is like normal peripheral vision, we also asked a normally-sighted observer to copy the same original but using only peripheral vision. While fixating steadily on the central fixation mark, “B”, the observer was asked to examine the original on the left, out of the corner of his eye, and to draw a copy on the right, all while maintaining fixation. Note that drawings A and B are similarly poor: The general object shape is preserved, and most details are present, but they are misplaced. These observers, with agnosic and normal vision, are not practiced artists, but their copies are limited more by perception than by motor skill. We can better assess how well they reproduced appearance by arranging to see the drawings as they did. In the case of the peripherally-viewing normal observer, you should fixate the letter B, between the two diagrams. While fixating the B, notice that the left and right diagrams are very similar, which shows that the normal observer did a good job of producing a perceptual match. The conjecture of this paper is that central agnosia is like peripheral crowding, so we provide a fixation point (A) that places the agnosic patient’s copy in your right peripheral visual field. We have set the eccentricity to make your peripheral vision equivalent to the agnosic’s central vision. The original diagram appears to the left of fixation at the same eccentricity. Again, when you now fixate on the A, you will find that the copy, seen peripherally, looks much like the original, also seen peripherally. We hope these informal demonstrations help you see that agnosia might be like your peripheral vision. Evidence is coming, in Results.
Figure 2. What they draw.
Each row presents the standard Rey (1941) Complex Figure (https://en.wikipedia.org/wiki/Rey%E2%80%93Osterrieth_complex_figure) on the left and a hand-drawn copy on the right. The A. copy was made by an apperceptive agnosic patient with unrestricted viewing (Lê et al., 2002). The B. copy was made by normal observer PMS, who was instructed to fixate on the mark (replaced here by the letter B), and never look away, while copying the original in his left periphery to the blank page in his right periphery. Please note, first, that both copies, viewed directly, seem poor, with many obvious errors. According to Caffarra et al. (2002) both copies are abnormal falling within the lowest 5% (A. copy raw score 26, corrected for age and schooling years 23.5; B. copy raw score 20, corrected 19.5). Then try to see them as the participants did, by fixating on the letter A or B. This simulates the vision of the agnosic observer in A, and replicates what the normal observer did in B. When the copies are viewed peripherally, we find that they are remarkably good. All figures were hidden during rest breaks. The agnosic copy in row A is from Lê et al. (2002). The normal-periphery copy in row B appeared previously, with our permission, in Pelli and Tillman (2008).
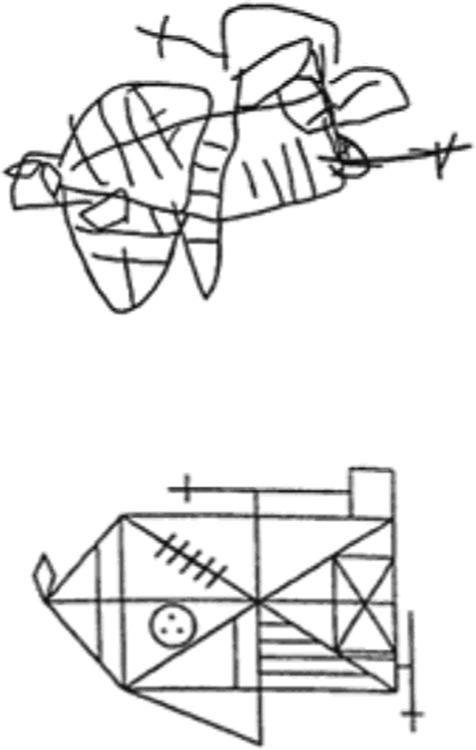
For comparison, Fig. 3 shows a copy made by an apraxic patient. It is very different from the peripheral and agnosic copies. Some details are preserved, but the overall shape is wrong.
Figure 3. Copying.
A copy of the standard Rey Complex Figure made by a patient with constructive apraxia (Loring et al., 1990). The copy is extremely poor with a raw score of 7.5 (corrected score for age and schooling years 6; Caffarra et al., 2002). It is very different from the agnosic and peripheral copies in Fig. 2. From Loring et al. (1990).
We conjecture that the agnosic patient directly viewing a complex display behaves like a normally-sighted observer viewing it peripherally. In both cases, according to our crowding conjecture, recognition is limited by the spacing of the simple objects making up the complex display. To test our conjecture, we took 14 screening tests from widely used batteries for the assessment of agnosic deficits. We presented them to the peripheral vision of a normally-sighted “standard” observer, at several eccentricities, ranging from 0 to 20 deg, and graphed performance as a function of eccentricity (Fig. 5a in Results).
Figure 5. Raw performance of the eccentrically-viewing normal observer (a) and the patients (b).
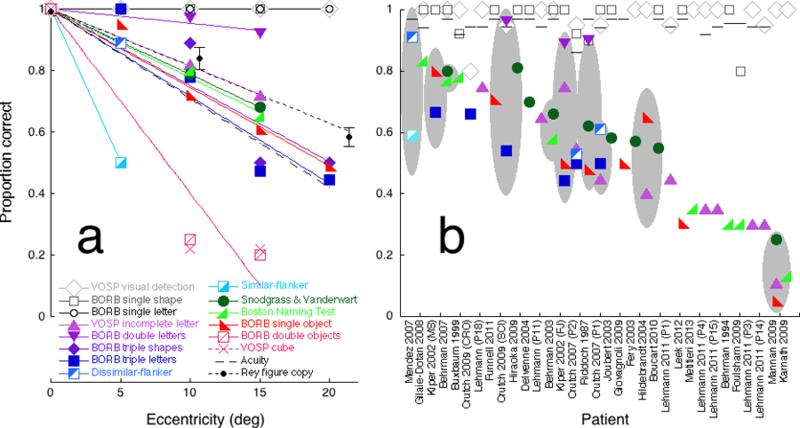
(a) Performance by normal observer PMS of each test as a function of eccentricity. Performance p is proportion correct on each test except acuity and the Rey figure copy, for which we plot an acuity index pacuity (dashed line) and a copy index (solid black line). (b) Published proportion correct (or acuity index) of each patient (or group) for each test. There are 32 individual patients and one group. The group consists of fourteen Posterior Cortical Atrophy patients (Mendez et al., 2007). The horizontal scale lists each study’s first author and year of publication, sorted by mean performance on complex-display tasks. For each patient (or group), the grey ellipse indicates the 95% confidence interval for the mean across the complex-display tasks.
This graph is a bit like a Rosetta stone, in the sense that it translates performance on various tests to one, the “equivalent eccentricity” in the normal vision of our standard observer, PMS. We then compared the peripheral performance accuracy of the standard observer with the previously reported individual accuracies of 32 agnosic patients and a group of 14 PCA patients with agnosic deficits, all selected from the literature. Each test for agnosia has its own scoring. We show that it can be helpful to convert each raw performance score to another number, the “equivalent eccentricity” of the patient’s performance. Equivalent eccentricity φeq for a particular patient and task is the eccentricity φ at which our standard observer performs the task equally well as the patient.
For use in later sections, note that, in normal vision, letter acuity size A and the crowding distance Scrowding both grow linearly with eccentricity φ,
| (1) |
| (2) |
and letter recognition is bounded by both limits (Song et al. 2014). The 0.17 deg offset in Eq. 2 has been updated in light of recent foveal measurements by Pelli et al. (2016)
2. METHODS
Overview
We took 14 widely used clinical tests from the neuropsychological batteries used for agnosic screening, and administered them to our normally-sighted “standard” observer at each of five eccentricities (0, 5, 10, 15, 20 deg). For each test, this yielded a graph of normal accuracy as a function of eccentricity, to which we fit a line, by least squares. Three of the tests are simple displays, which are immune to crowding. They use large symbols, within the acuity limit, so performance is independent of eccentricity (slope zero in Fig. 5a and Table 2 in Results). Single-letter acuity worsens with eccentricity, even though it is immune to crowding (Fig. 5a). The rest of the tests are complex displays, which are susceptible to crowding, and performance depends on eccentricity (nonzero slope in Fig. 5a and Table 2). For each eccentricity-dependent test, the line assigns an equivalent eccentricity to each level of performance. We used those lines, based on the standard observer’s performance, to transform all the patients’ data. For each patient, for each eccentricity-dependent test, we converted the test score to an equivalent eccentricity.
Table 2. Equivalent eccentricity conversion.
| (7) |
| (8) |
| Symbol | Test | Slope m (deg−1) | Stimulus |
|---|---|---|---|

|
Similar flanker | −0.100 |
|

|
VOSP cube | −0.060 |
|

|
BORB double objects | −0.059 |
|
|
|
BORB triple letters | −0.030 |
|

|
BORB triple shapes | −0.025 |
|
|
|
BORB single objects | −0.025 |
|

|
Dissimilar flanker | −0.022 |
|

|
Boston Naming Test | −0.022 |
|

|
Snodgrass & Vanderwart | −0.020 |
|
|
|
VOSP incomplete letter | −0.020 |
|
|
|
BORB double letters | −0.005 |
|

|
VOSP visual detection | −0.000 |
|
|
|
BORB single shape | −0.000 |

|
|
|
BORB single letter | −0.000 |
|
|
| |||
|
|
Acuity | −0.029 |

|
According to our crowding conjecture, objects and tasks that are immune to crowding will be spared by apperceptive agnosia. Tasks and objects that are immune to crowding include detection of any shape, judging orientation of horizontal vs. vertical lines, recognition of an isolated letter or digit, and single-letter identification and acuity (Pelli et al., 2004). On the other hand, for crowding-susceptible complex displays, the degree of impairment for each apperceptive agnosia patient should be fully predicted, for all tasks, by the performance of our standard observer at some equivalent eccentricity.
Participants: Literature search and inclusion criteria
3763 papers published between 1900 and 2013 were found by searching the PubMed and Google Scholar databases for visual agnosia using the keywords listed in Fig. 4, and checking the reference lists of the identified papers. This included some papers on Posterior Cortical Atrophy (PCA) patients who show a perceptual deficit identified as apperceptive agnosia (McMonagle, Deering, Berliner, Kertesz, 2006). [The Yong et al. (2014) study of 26 PCA patients appeared too late to be included in our sample.] On the basis of the title and abstract, papers describing cases of associative agnosia or associative prosopagnosia were excluded, as well as case descriptions of Klüver-Bucy syndrome and Alzheimer patients with semantic deficits, and further papers reporting the same case. This yielded 58 papers, which were fully assessed. 34 papers were excluded from further investigation because either 1) no data were reported on standard agnosia tests, or 2) the reported visual acuity indicated a deep impairment. The 24 papers included in the meta-analysis are listed in Table 1. 15 patients from these studies were excluded: 14 from the study of Lehmann et al (2011) because of deeply impaired acuity; and 1 patient (JJ) from the study of Mannan et al. (2009) for having symptoms related to simultanagnosia1. Figure 4 presents a flowchart of the patient selection process.
Figure 4. Flowchart of the patient selection process.
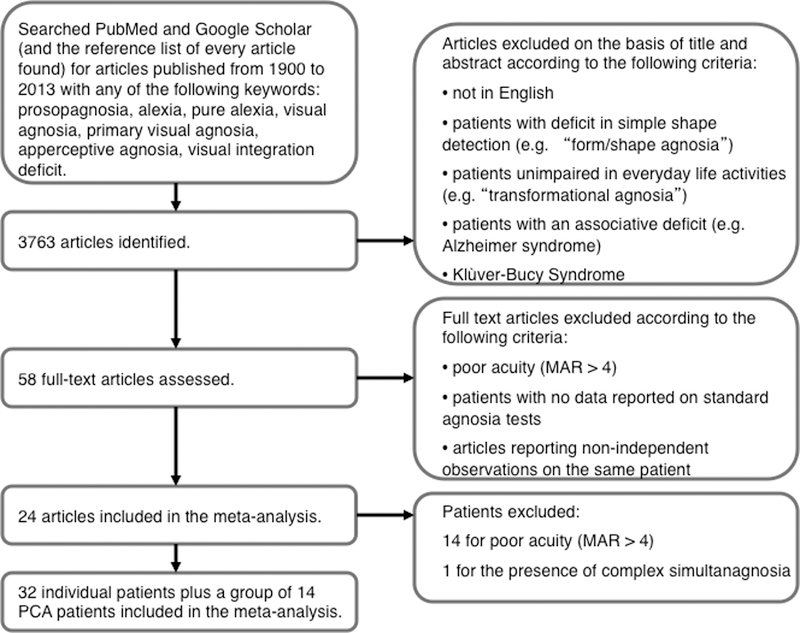
In the chart, MAR (minimum angle of resolution) indicates the angle (in minarc) which the strokes of the letter subtend at the person’s eye.
Table 1.
The 32 individual patients and the group of fourteen Posterior Cortical Atrophy (PCA) patients taken from the literature, as explained in Methods: Participants. For each patient (and the PCA group) the equivalent eccentricity column specifies the patient’s (or group’s) mean equivalent eccentricity for complex displays in Fig. 5.
| Case | Sex | Age | Lesion | Etiology | Eq. ecc. |
|---|---|---|---|---|---|
| Behrmann et al. (1994) C.K. | M | 33 | unknown | motor vehicle accident | 32 |
| Behrmann & Kimchi (2003) S.M. | M | 22 | right anterior and posterior temporal regions, corpus callosum and left ganglia | head injury | 18 |
| Behrmann & Williams (2007) C.R. | M | 16 | right temporal lobe lesion and microabcesses of the right temporal and medial occipital lobe | right temporal brain abscess | 10 |
| Buxbaum et al. (1999) W.B. | M | 47 | unknown | large bilateral posterior intraparenchymal hemorrhage | 10 |
| Boucart et al. (2010) W.S. | F | 57 | bilateral atrophy of the parieto-occipital lobes | posterior cortical atrophy | 21 |
| Crutch & Warrington (2007) P1 | F | 74 | unknown | posterior cortical atrophy | 20 |
| Crutch & Warrington (2007) P2 | F | 58 | unknown | posterior cortical atrophy | 19 |
| Crutch & Warrington (2009) C.R.O. | N/A | 59 | mild loss of cerebral cortical volume, no focal lesion | posterior cortical atrophy | 11 |
| Crutch & Warrington (2009) S.C.I. | N/A | 70 | posterior cortical atrophy in the occipitoparietal cortex | posterior cortical atrophy | 12 |
| Delvenne et al. (2004) N.S. | M | 40 | bilateral occipito-temporal junction and left parietal and frontal sites | car accident | 14 |
| Fery & Morais (2003) D.J. | M | 59 | left occipital lesion | left posterior cerebral artery stroke | 20 |
| Foulsham et al. (2009) C.H. | F | 63 | unknown | posterior cortical atrophy | 32 |
| Funnell & Wilding (2011) S.R. | F | 9 | bilateral attenuation in the temporal regions primarily right | encephalitis | 12 |
| Gilaie-Dotan et al. (2009) L.G. | M | 19 | unknown | developmental object agnosia and prosopagnosia | 8 |
| Giovagnoli et al. (2009) R.M. | F | 64 | unknown | slowly progressive visual agnosia | 20 |
| Hildebrandt et al. (2004) A.M. | M | 46 | unknown | heart arrest | 21 |
| Hiraoka et al. (2009) | F | 74 | right occipital, right half of the splenium of the corpus callosum extending forward to the pulvinar | posterior cerebral artery stroke | 12 |
| Joubert et al. (2003) F.G. | M | 71 | unknown | slowly progressive visual agnosia | 20 |
| Karnath et al. (2009) J.S. | M | 74 | bilateral medial ventral occipitotemporal cortex | ischemic stroke | 40 |
| Kiper et al. (2002) F.J. | M | 18 | bilateral symmetric occipital hypodensities | hemophilus influenzae | 18 |
| Kiper et al. (2002) M.S. | F | 7 | right occipital and no left occipital cortex | bacterial meningitis | 10 |
| Lehmann et al. (2011) P1 | M | 69 | unknown | posterior cortical atrophy | 25 |
| Lehmann et al. (2011) P3 | F | 64 | unknown | posterior cortical atrophy | 32 |
| Lehmann et al. (2011) P4 | M | 49 | unknown | posterior cortical atrophy | 30 |
| Lehmann et al. (2011) P11 | F | 63 | unknown | posterior cortical atrophy | 16 |
| Lehmann et al. (2011) P14 | M | 60 | unknown | posterior cortical atrophy | 32 |
| Lehmann et al. (2011) P15 | F | 70 | unknown | posterior cortical atrophy | 30 |
| Lehmann et al. (2011) P18 | F | 51 | unknown | posterior cortical atrophy | 11 |
| Leek et al. (2012) I.E.S. | M | 78 | bilateral ventral-occipital, left lingual gyrus, the fusiform gyrus bilaterally | posterior cerebral artery stroke | 28 |
| Mannan et al. (2009) S.F. | F | 52 | unknown | posterior cortical atrophy | 38 |
| Metitieri et al. (2013) L. | M | 12 | MR high intensity signal in the left parietooccipital and calcarine sulci with atrophy of the occipital lobe | lethargy, hypotony, and convulsions | 30 |
| Riddoch & Humphreys (1987) H.J.A. | M | 61 | bilateral inferior temporal gyrus, lateral occipitotemporal gyrus, the fusiform gyrus and the lingual gyrus | posterior cerebral artery stroke perioperatively | 20 |
| Mendez et al. (2007) fourteen PCA patients | M&F | 53–72 | unknown | posterior cortical atrophy | 4 |
Each candidate patient was included only if he or she met all three of the following criteria:
1. Patient preserves elementary visual abilities. Patients with visual fields defects, and or those not able to solve a shape-detection task (e.g., the VOSP screening test, see below for a description) were not included in the analysis.
2. Patient’s visual recognition is poor enough to impair everyday life activities. In some patients, the deficit is confined to the recognition of objects drawn or photographed from an unusual perspective. Such patients are classified as “transformational agnosic” (Humphreys & Riddoch, 1987; Warrington, 1985). Their impairment has been interpreted as a categorization deficit (Farah, 2004), or as a perceptual transformation inability (Warrington and James, 1988; Warrington and Taylor, 1973). Warrington (1985) locates this agnosic deficit high in the perceptual hierarchy, suggesting that it impairs the perceptual computation used to transform the visual input, occluded or seen from an unusual perspective, to its prototypical form stored in memory. In any case, interpretation of that deficit goes beyond our scope here.
3. The patient’s accuracy is reported for at least one standardized agnosia test that uses a complex display. We included those patients who have been tested in at least one simple-display agnosia test (visual detection, single geometric shape, and single letter) and at least one complex-display agnosia test: single-drawing identification, double-drawing identification, double-letter identification, triple-letter identification, crowding test with similar/dissimilar flankers, triple-geometric shape identification, and cube analysis.
We excluded simultanagnosia from our sample because it is unlike visual agnosia and seems to be an attentional rather than a perceptual deficit (Coslett and Saffran, 1991). Simultanagnosia patients recognize single parts of a complex display, but fail to recognize the whole. Moreover, patients with simultanagnosia can recognize single complex objects (e.g. a word), if presented in isolation, and in such testing their shape perception is intact.
This process yielded 24 papers, reporting 46 patients: 32 individual patients and a group of 14 PCA (Posterior Cortical Atrophy) patients (Table 1). Within this harvest of the apperceptive agnosia literature, 10 individual patients and the PCA group each took multiple tests using complex displays. More specifically, 22 patients took one simple and one complex test; 6 individual patients and the PCA group took one simple and two complex tests; and 4 patients took one simple and three complex tests.
The neurologically intact standard observer (PMS) was selected to have the same level of education (eighteen years) and age as HJA (one of the most-tested visual agnosic patients) at the time of testing. PMS was 61 years old, had no important ophthalmological history at the time of testing, having mild myopia with visual acuity corrected to normal. As far as we know, normal adult vision would have sufficed to get our results. Three of the tests administered to PMS were also presented to 8 normally sighted university students of the Psychology Department at the University of Rome La Sapienza (mean age 26.5 years; 6 male and 2 female).
Stimuli
We selected 14 tests for the assessment of agnosic deficit, plus an acuity test, for a total of 15. We administered the following 10 visual tests taken from two widely used screening batteries (VOSP and BORB) and four additional object recognition tests to the standard observer: VOSP (visual detection test, cube analysis, and incomplete letter), BORB (single-, double- and triple-letter identification, single- and triple-geometric shape identification and single- and double-object identification), figure identification (from Snodgrass & Vanderwart, 1980, and from The Boston Naming Test, Goodglass et al., 1983) and two tests with similar and dissimilar flankers (from Mendez et al., 2007). When double or triple items were presented (BORB double objects and triple shapes), we asked the normally-sighted observer to identify all items, and we counted the response as correct only if all the items were correctly reported, regardless of order.
The selected images were scanned. For each trial, one image was presented in the center of the screen. Two classes of stimuli were used: simple and complex. The simple images (and associated task) are immune to crowding. The complex images (and associated tasks) are susceptible to crowding. Each simple image is a single uppercase letter, a geometric shape, or a simple figure. Each simple image subtended 4.5 deg horizontally and vertically. Each complex image consisted of two or three simple objects side by side (e.g. triple-letter identification). Each double image subtended 9 deg horizontally, and each triple image subtended 13.5 deg horizontally.
Acuity
—— Acuity
[Each test name is followed by the symbol used to represent it in the figures.]
Measured acuity depends on details of the test and procedure. In standard clinical testing of neuropsychological patients, “normal” acuity corresponds to a Minimum Angle of Resolution (MAR) M of 1 minute of arc, which is the highest acuity tested. They do not test smaller letters. Acuity letter size A, in deg, is proportional to the MAR, which is in minutes of arc,
| (3) |
Normal acuity size grows linearly with eccentricity (Eq. 1), which we solve for eccentricity, to obtain a formula that converts acuity to equivalent eccentricity for acuity,
| (4) |
where A is letter acuity size. The nominally normal acuity of 1 minarc MAR (i.e. A = 0.0833 deg) has an equivalent eccentricity of 0.15 deg. Patient MAR acuities ranged from 1 to 4 minarc, so, by Eq. 4, their equivalent eccentricities for acuity ranged from 0.15 to 8.8 deg.
Three simple crowding-immune tests
Shape detection screening test:  VOSP visual detection
VOSP visual detection
Our VOSP visual detection test is the shape-detection screening test in the Visual Object and Space Perception (VOSP) battery (Warrington and James, 1991), which evolved from the form test (figure-ground discrimination) of Warrington and Taylor (1973). The task was to detect (yes/no) whether an X is present in a field of binary noise, 50% white and 50% black. If present, the region of the X had a higher proportion of white than black. The X was present in half of the 20 trials. The standard observer was presented with the set of 20 trials for each eccentricity tested, and was asked to detect the presence of the X. As specified by the authors of the test, texture density was not considered in the response scoring.
Identification:  BORB single shape,
BORB single shape,  BORB single letter
BORB single letter
These two tests are part of the Birmingham Object Recognition Battery (BORB) (Riddoch & Humphreys, 1993). On each trial, the observer identified an object. In the BORB single shape task, there were 36 trials showing one out of seven different geometric shapes (circle, triangle, square, pentagon, hexagon, Greek cross, Greek cross rotated by 45°). In the BORB single letter task, there were 36 trials, each presenting one out of 12 possible uppercase letters (A, C, D, G, H, L, J, M, R, S, U, and V).
Eight complex crowding-susceptible tests
 VOSP incomplete letter
VOSP incomplete letter
The VOSP incomplete letters task was developed by Warrington and James (1991) and was included in the VOSP. The observer was asked to identify an uppercase letter that has been “degraded” by omitting fragments. There were 21 uppercase letters (including a practice trial) degraded by 30% to 70%. Letter identity, ordered by increasing degradation, is: F, B, P, D, V, M, S, K, X, Y, H, C, Z, A, E, L, G, U, R, W, and N. At each eccentricity, as specified by the authors of the test, we presented one letter per trial, for 21 trials, the first of which was practice, and scoring total accuracy on the non-practice trials regardless of degradation.
Identification of single drawing:  BORB single object,
BORB single object,  Boston naming test,
Boston naming test,  Snodgrass & Vanderwart
Snodgrass & Vanderwart
In these three tasks, the observer identified the drawing shown on each trial. BORB single object, taken from BORB, had 40 drawings of everyday objects, animals, and plants. In the Boston Naming Test (BNT), there were 30 drawings of familiar objects (e.g. helicopter, octopus, comb) (Kaplan et al., 1983). Finally, the Snodgrass & Vanderwart (1980) test had 260 line drawings of everyday objects. The standard observer was presented with the three tests in separate blocks for a total of 330 trials at each eccentricity.
Double-letter identification:  BORB double letters
BORB double letters
Also from BORB, on each trial, the observer identified a pair of letters. There were 36 trials. The two letters were each taken from the same set of possible letters used in the BORB single letter test. The observer was asked to identify both letters, and the response was scored as correct only if both letters were correctly named, in any order.
Triple-letter identification:  BORB triple letters
BORB triple letters
Also from the BORB, on each trial, the observer identified the three letters presented, in any order. There were 36 trials, and the three letters in each trial were selected to be always different. The response was scored as correct only if all three letters were correctly named, in any order.
Letter strings:  Similar flanker,
Similar flanker,  Dissimilar flanker
Dissimilar flanker
Mendez et al. (2007) used this test with patients affected by Posterior Cortical Atrophy. The task was to read the central letter of a letter triplet, ignoring the flankers. There were 56 trials, in random order. On 28 trials, the flanking letters were similar to the central target letter (Similar flanker), and, on the other 28 trials, they were not similar to the target letter (Dissimilar flanker). Crowding studies have shown that similar flankers produce more crowding than dissimilar flankers do.
Three more crowding-susceptible tests
For future estimation of equivalent eccentricity in neuropsychological patients, Table 2 also reports normal results on three more tests for which we did not find any patient results to present here. Since there is no patient data, these tests appear only in Table 2, not in any of the figures.
Triple geometric shapes:  BORB triple shapes
BORB triple shapes
Also from BORB, the observer was asked to identify three shapes. 36 trials each presented three different shapes sampled from a set of seven (circle, triangle, square, pentagon, hexagon, Greek cross, Greek cross rotated by 45°). The response was scored as correct only if all three shapes were correctly identified, in any order.
Identification of double drawings:  BORB double objects
BORB double objects
Also from BORB, the observer was presented with two drawings, side by side, and asked to identify both. This test consisted of 40 double drawings of everyday objects, animals and plants. The response was correct only if both items were correctly identified, in any order.
Cube analysis:  VOSP cube
VOSP cube
This test of visuo-spatial abilities is part of the VOSP battery (Warrington and James, 1991). The observer was asked to say how many cubes were depicted in a line drawing. The observer performed 2 practice trials and 10 test trials.
Apparatus and procedure
Stimuli were presented on a LaCie 21-inch monitor driven by a Power Mac G5 computer. The monitor was 57 cm in front of the observer’s eyes. The experiment was implemented in MATLAB software with the Psychophysics Toolbox extensions (http://psychtoolbox.org; Brainard, 1997; Pelli, 1997).
The monitor was directly in front of the seated standard observer (PMS), and the observer was asked to face the display, moving only his eyes to fixate the static black cross constantly present to the left of center of the display. The observer was asked to visually fixate the black cross constantly. While fixating, he was asked to identify an image that appeared in the center of the screen for 200 ms. The tests were conducted at five eccentricities, 0, 5, 10, 15, 20 deg, by varying the distance of the black cross from the center of the screen. At 0 deg of eccentricity (direct view), the fixation mark disappeared 100 ms before stimulus onset. The responses were recorded by the experimenter using one button for correct and another for incorrect. Recording the response initiated the appearance of the next stimulus. Vocal responses supplied by the observer were also audio-recorded for offline review after testing. The observer was encouraged to respond accurately and to describe the perceptual experience even in those cases in which he was not able to correctly identify the stimulus. PMS was also asked to describe verbally and copy stimuli seen in his periphery. PMS took part in eight experimental sessions of one hour each, over a three-month period.
Eight more normal observers were tested in two different experiments on three tasks (Similar flanker, Dissimilar flanker, Single Letter, as defined above) in a single session with the same procedure used for PMS. The letter x-height was 5 deg. (This is negligibly larger than the 4.5 deg size used with PMS.) The target letter was presented in central vision, either alone, or between two flanker letters, which were either similar or dissimilar to the target letter. For one experiment, we measured accuracy as a function of eccentricity (0, 4, or 8 deg), to compare with PMS. For the other experiment, using central viewing, we measured accuracy for the three tasks as a function of blur (pillbox radius: 0, 1.41, 2.82, 3.52, 4.22 or 5.63 deg). Stimuli were blurred with the MATLAB function “fspecial”, using the disk option to specify the diameter the averaging filter (pillbox). Each of the 3×(6+3) conditions (no, similar, and dissimilar flanker; six degrees of blur; and 3 eccentricities) was tested for 20 trials, for a total of 540 trials in one long session.
3. RESULTS
We tested our conjecture that agnosic central vision is like normal peripheral vision. To that end, we compared performance accuracy of the eccentrically viewing standard observer with the previously reported central performance of 46 patients with visual agnosia (32 individuals and a group of 14 PCA patients, see Table 1).
We have several layers to peel off in examining the results. First is the dichotomy between simple and complex displays. Simple displays are immune to crowding and show little or no effect of eccentricity, whereas complex displays are susceptible to crowding and are much harder to identify at greater eccentricity. This dichotomy is an important similarity between the phenomena of crowding and apperceptive agnosia: Both deficits spare identification of simple displays and impair identification of complex displays. Our strongest evidence for crowding is the stunning regularity of the complex-display results. Apperceptive agnosia and crowding are similar to each other in how they affect the whole gamut of complex displays. As explained below, this regularity is manifest by finding that each patient conserves “equivalent eccentricity” across tasks. Furthermore, at the end of Results, comparing results from agnosia and eccentricity, we will find that the relative susceptibility of the tasks to crowding is conserved in agnosia.
Comparing peripheral and agnosic vision
Figure 5a plots the standard observer’s performance on each of the 14 different tests (each indicated by a different geometric symbol) as a function of eccentricity. Three tasks used simple displays (open symbols): visual detection (of X in texture), identification of single geometric shapes, and identification of single letters. Twelve tasks used complex displays (filled symbols): identification of an incomplete letter, identification of single and multiple drawings of objects, identification of two or three letters, and identification of a target letter in the presence of two nearby similar or dissimilar flankers, triple geometric shapes, and the cube test. Performance of large simple-display tasks was unaffected by eccentricity (open symbols) and performance of complex-display tasks dropped rapidly with eccentricity (filled symbols). In normal peripheral vision, complex object recognition is limited by crowding, which grows with eccentricity, while perception of a large simple image is unaffected by eccentricity.
In normally sighted observers, acuity size grows linearly with eccentricity (Eq. 1). In order to plot acuity on our 0 to 1 “performance” scale, and have it drop with eccentricity, we offset acuity size A to produce an “acuity index” pacuity,
| (5) |
which is the dashed line in Fig. 5a.
PMS’s copy of the Rey complex figure while viewing peripherally is much like the agnosic patient’s copy (Fig. 2). To evaluate the dependency of the Rey test score on viewing eccentricity, we asked 10 new observers to copy the Rey figure, each at just one eccentricity (3 observers at 0 deg, 3 and PMS at 11 deg, and 4 at 21 deg) to avoid contamination of our results by any learning of the Rey figure at another eccentricity. The scoring rules assign zero only when there is no attempt to copy, so our raw scores have a minimum of 1, and we normalize the log score log s by its highest possible value, log S, to produce a “copy index” pcopy that ranges from 0 to 1,
| (6) |
The drop in performance with eccentricity for copying the Rey figure (black disks in Fig. 5a) is similar to those for recognition of complex displays. However, performance of this copying task is dominated by personal drawing ability, not perception, so we do not report equivalent eccentricities for copying.
Figure 5b plots the published patients’ performance of the same 14 tests and acuity. The patients are sorted by mean accuracy on complex-display tasks. Each study’s first author and year are indicated beneath the horizontal axis. Each column of symbols represents an individual patient, except the first column, which represents the group of fourteen PCA patients reported by Mendez et al. (2007). The three (large) simple-display tasks (open symbols) were unaffected by eccentricity (Fig. 5a) and agnosia (Fig. 5b). Like the large simple-display tasks, acuity (Fig. 5b line symbol) is unaffected by agnosia. Performance of the twelve complex-display tasks (filled symbols) was severely impaired by increasing eccentricity in the standard observer (Fig. 5a), and showed low performance and considerable variability across patients (Fig. 5b). For each patient or group of patients who did several complex-display tasks, the grey ellipse indicates the 95% confidence interval about the mean score (Fig. 5b).
Table 2 presents the slopes of the linear regression of performance vs. eccentricity (p vs. φ) for each task performed by the standard observer, PMS. The slopes m are used to calculate equivalent eccentricity (see below). For use in future studies, we list results for all the tests that PMS performed, including three that do not appear in Fig. 5b because none of the included patients took those tests.
In normally sighted observers, the crowding distance (the letter spacing needed to reach a criterion level of task performance) grows linearly with eccentricity, with a proportionality constant b that is related to the slope m of accuracy vs. eccentricity (Eq. 2 sets b = 0.3). There is some variation of b across individuals (Toet and Levi, 1992).
Normal variation.
We measured the variability of m across normal individuals. We collected data on eight new normally-sighted observers on three of the tasks administered to PMS. We chose  Similar flanker and
Similar flanker and  Dissimilar flanker, which have very different slopes, and we included the corresponding no-flanker (
Dissimilar flanker, which have very different slopes, and we included the corresponding no-flanker ( BORB single letter) condition. The regression lines (not shown) for proportion correct vs. eccentricity are good fits, with a median R2 of 0.87 (range 0.63 to 0.99) with flankers and 0.7 (range n.s. to 0.7) without flankers. The mean ± standard deviation, across observers, of the slope is −0.08±0.01 for the similar-flanker and −0.03±0.01 for the dissimilar-flanker condition, a nearly threefold reduction, and nearly zero (−0.006 ± 0.003) for the no-flanker condition. The estimated slopes for observer PMS (−0.100 similar flanker; −0.022 dissimilar flanker, 0.000 no flanker) lie within the range (not shown) of those of the new observers. Randomly selecting one of these normally-sighted observers to be the standard observer, to calculate equivalent eccentricity, would perturb the estimated equivalent eccentricities with a standard deviation of 33% (0.01/0.03) or less about the mean value. However, this paper is more concerned with the difference between tasks. The mean ± standard deviation across observers of the ratio of slope with similar-flanker over that for dissimilar-flanker is 2.7 ± 0.9. Thus the accuracy-vs.-eccentricity slope m varies little across observers (at most 33%) and hugely across tasks (270%).
BORB single letter) condition. The regression lines (not shown) for proportion correct vs. eccentricity are good fits, with a median R2 of 0.87 (range 0.63 to 0.99) with flankers and 0.7 (range n.s. to 0.7) without flankers. The mean ± standard deviation, across observers, of the slope is −0.08±0.01 for the similar-flanker and −0.03±0.01 for the dissimilar-flanker condition, a nearly threefold reduction, and nearly zero (−0.006 ± 0.003) for the no-flanker condition. The estimated slopes for observer PMS (−0.100 similar flanker; −0.022 dissimilar flanker, 0.000 no flanker) lie within the range (not shown) of those of the new observers. Randomly selecting one of these normally-sighted observers to be the standard observer, to calculate equivalent eccentricity, would perturb the estimated equivalent eccentricities with a standard deviation of 33% (0.01/0.03) or less about the mean value. However, this paper is more concerned with the difference between tasks. The mean ± standard deviation across observers of the ratio of slope with similar-flanker over that for dissimilar-flanker is 2.7 ± 0.9. Thus the accuracy-vs.-eccentricity slope m varies little across observers (at most 33%) and hugely across tasks (270%).
Crowding seems to be highly conserved across adult age. A recent study found no change in the crowding distance over the adult age range of 18 to 76 years (Astle, Blighe, Webb, & McGraw, 2014). This indicates that the standard eccentricity dependence documented in Table 2 is independent the standard observer’s age. Indeed, we found that the slopes for PMS, who was 61 years old, are similar to those of eight students in their twenties.
PMS is our standard observer. The parameters of his vision (Table 2), allow raw performance scores on any of the 14 neuropsychological tests to be mapped into a standard scale: equivalent eccentricity of viewing by our standard observer PMS. This standard scale makes it easy to compare across tests and patients, to determine whether a patient’s equivalent eccentricity is conserved across tests, and to compare the severity of agnosic deficit across patients.
This use of a single human being to create a standard coordinate space for future studies of many people is in the same spirit as the popular use of Talairach coordinates, based on dissection of a single human brain, to indicate the location of brain structures (Talairach & Tournoux, 1988).
In visual agnosia, equivalent eccentricity is conserved and equivalent blur is not
We used Eq. 7 and Table 2 to convert each test score to the equivalent eccentricity, i.e. the eccentricity at which the standard observer would perform that test as poorly as the directly-viewing patient. The crowding conjecture predicts that each patient has the same equivalent eccentricity on all tests, i.e. equivalent eccentricity is conserved. Thus, each patient’s deficit is entirely characterized by this number. Tests that are independent of eccentricity (slope zero in Table 2) are also unaffected by apperceptive agnosia.
Figure 6 shows all the equivalent eccentricities for each patient. The equivalent eccentricity (vertical scale) indicates the severity of the agnosic deficit. In normal peripheral vision, crowding distance increases linearly with eccentricity, so larger equivalent eccentricity predicts larger crowding distance, i.e. a need for greater separation of target from clutter, in central agnosic vision.
Figure 6. Each patient has a consistent equivalent eccentricity, across all complex-display tests.
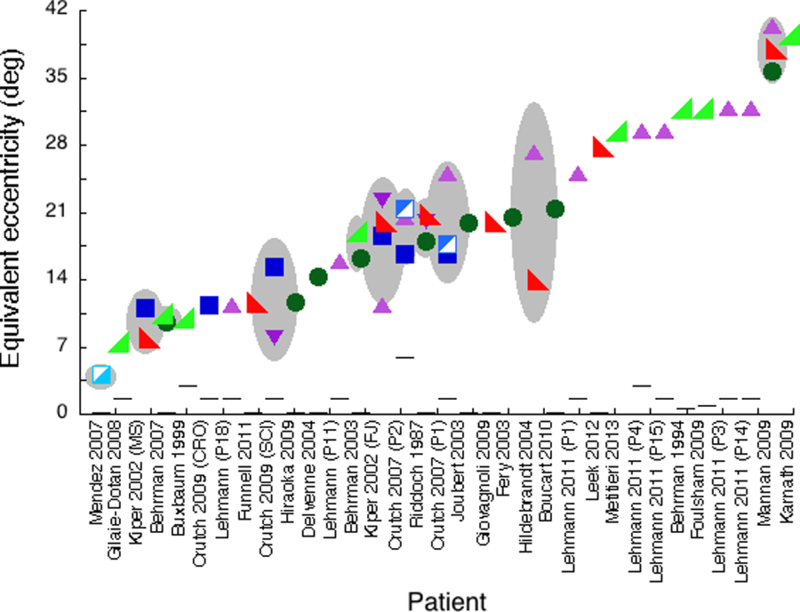
For each patient, the figure presents the equivalent eccentricity for each test score for every eccentricity-dependent test. Overall, there are 32 individual patients and a group of fourteen PCA patients, described in 24 papers. For 10 individual patients and the PCA group (Mendez et al. 2007) we have performance on multiple complex displays; this is indicated by the presence of more than one symbol in a column and a gray ellipse, whose vertical extent indicates the 95% confidence interval across tests. The various space-filling symbols are for complex-display tasks. The horizontal-line symbols are for acuity, which is a simple-display task.
Figure 6 shows good conservation of equivalent eccentricity across tests. We have accuracy on at least two eccentricity-dependent tests for 10 individual patients and the group of PCA patients. For each patient, the wide range of raw performance in Fig. 5b corresponds to practically a single eccentricity in Fig. 6.
In a pairwise comparison of all complex-display performances across tests, the correlation of proportion correct (r = 0.56, p < 0.01) is much weaker than that of equivalent eccentricity (r = 0.83, p < 0.0001). Across tests, the equivalent eccentricity is much more consistent than the raw performance score. Each patient’s equivalent eccentricity is conserved across tasks.
Our finding that equivalent eccentricity is conserved across tests comes from the patients who took multiple complex-display tests: 10 individual patients and the PCA group. Fig. 6 shows they are typical: The equivalent eccentricities of the patients with multiple tests are typical of the whole study sample. The mean ± SD equivalent eccentricity is 18 ± 9 deg for patients who took multiple, and 22 ± 9 deg for patients who took a single complex-display test. Thus there is no significant difference in the severity of the agnosia between the patients who took single vs. multiple tests.
Is the crowding impairment independent of visual acuity? Visual acuity size, like the crowding distance, also increases linearly with eccentricity. Figure 6 shows visual acuity estimates reported for each patient converted to equivalent eccentricity (line symbols). The equivalent eccentricities for acuity are far better than those for all other tests and independent of the severity of the agnosia. The mean ± SD of equivalent eccentricity for acuity across 25 patients and the PCA group is 1.19 ± 1.31 deg. For two patients, FJ and MS, the corrected visual acuity is not reported numerically (Kiper et al., 2002), but the authors affirm that, “In both patients basic visual functions visual acuity, contrast sensitivity, color, form, motion perception are similarly preserved or modestly impaired.”
Would any graded visual impairment produce the same result? J. A. Movshon (personal communication) and an anonymous reviewer wondered whether equivalent blur, like eccentricity, might also be conserved across tasks. That is worth checking, and the answer is no. We evaluated the performance decline of eight normal observers as a function of blur for three tasks (Similar flanker, Dissimilar flanker, and BORB single letter) that yielded a large range of accuracies for both PMS and the patients. Regression lines for accuracy vs. blur have a median R2 of 0.90 (range 0.62 to 0.99). Across the eight observers, for each task, the mean±SD slopes are: −0.18±0.02 for similar-flanker; −0.21±0.02 for dissimilar-flanker; and −0.22±0.02 for single-letter. Thus, the three tasks, one simple and two complex, all have the same dependence on blur (no significant difference), though they depend very differently on eccentricity, for which the slope is zero for single-letter, small but nonzero for dissimilar-flanker, and large, 4.5 times larger, for similar-flanker (Table 2). Equivalent blur (i.e. the blur at which our normal-sighted observers would perform as poorly as the patients) calculated for the Mendez at al. (2007) group shows a large difference across the complex-displays (equivalent blur: similar-flanker 2.28 deg; dissimilar-flanker 0.43 deg). Object recognition is multidimensional, so one cannot expect just any graded visual degradation to affect all tasks similarly. Unlike equivalent eccentricity, equivalent blur is not conserved across tests.
Could the deficit in apperceptive agnosia be explained by another low-level visual phenomenon, other than crowding? In accounting for these data, we rule out acuity and blur as mediating factors in agnosic vision, because patients have normal acuity, and blur lacks the needed task-dependence. None of the many other well-known visual interference effects is compatible with the agnosic data. Internal noise might be higher in these brain-damaged patients, but it would affect simple and complex targets similarly, unlike the data. Masking and contour interaction depend on overlap and decrease rapidly when masker-target spacing is increased beyond contiguity, unlike these data. Song et al. (2014, Eq. 3) find that masking extends beyond the target a distance of only 1.4 times acuity. Neuropsychological tests for agnosia use large objects, and agnosic patients have near normal acuity, so the gap between target and flankers is a large multiple of acuity. Thus, the agnosic deficit with these targets cannot be due to masking. Of the well-known visual interference phenomena, only crowding matches the agnosic data.
In sum, the patient’s equivalent eccentricity predicts his or her performance on every complex-display task. Complex-display tasks are limited by crowding, and simple-display tasks are not.
Another way to compare the effects of agnosia and eccentricity.
Above, we found a linear relation between proportion correct and eccentricity (Eq. 7) for all the tests (Fig. 5a). Table 2 reports the slope m of each test, which is its (negative) susceptibility to eccentricity. We now show that one can similarly estimate each test’s susceptibility to agnosia. If agnosic is like eccentric vision in impairing test performance, then one would expect the tests to have the same relative susceptibilities to agnosia and eccentricity. Alternatively, if agnosia and eccentricity limit vision in different ways then we would expect the diverse test objects to have different patterns of sensitivity to agnosia and eccentricity.
We can rewrite our eccentricity performance model (Eq. 7) as
| (9) |
where P is proportion correct, t designates which test, φ is the eccentricity, st is susceptibility of test t (called “-m” in Eq. 7), is the standard observer’s susceptibility at eccentricity φ, is the residual error of the model, and is the floor function. We set , use our measured values of , and ask the Excel Solver Add-in to solve for the test susceptibilities st (for all ten tests) that minimize the mean square error plus a regularizer, . The fit is good, with RMS error fitting 41 data points with 10 degrees of freedom.
In the same spirit, our agnosia performance model is
| (10) |
where sa is the susceptibility of agnosic observer a. We use the published values of P(t, a) and ask Excel to solve for the agnosic and the test susceptibilities sa and st that minimize the mean square error plus a regularizer, . Again, the fit is good, with RMS error 0.026 fitting 87 data points with 10–1+33 = 42 degrees of freedom.
The regularizers R1 and R2 impose a minimum of 0.003 on the test susceptibilities, and set the mean test susceptibility for the agnosic data to 0.025, which is the value found for the eccentric data.
| (11) |
| (12) |
Comparing the susceptibilities, estimated separately for agnosia and eccentricity, reveals that they are practically equal, with a correlation of 0.97 (Fig. 7). Thus the recognisability of these diverse test images is very similarly affected by agnosia and eccentricity.
Figure 7. Susceptibility to agnosia vs. susceptibility to eccentricity.
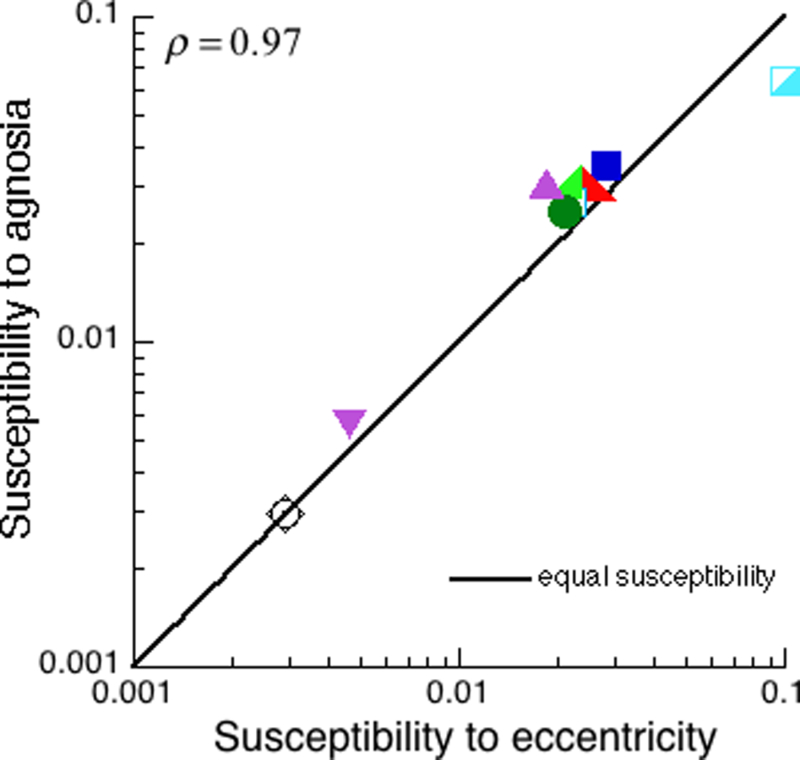
These are the ten tests for which we have both eccentric and agnosic patients’ data. Each test is represented by a point in the scatter diagram of susceptibility to agnosia sa vs. susceptibility to eccentricity . Susceptibility to eccentricity was computed solely from the performance of the normal observer viewing at many eccentricities. Except for an overall scale factor, susceptibility to agnosia was computed solely from the performance of the many agnosic patients using central vision. The points are near the equality line, showing relative susceptibility of the tests is similar, whether we look across diverse agnosias or eccentricities. On these log scales, the correlation is 0.97, and the RMS deviation from equality is . Once again, the effect of agnosia on object recognition is like the effect of eccentricity.
4. DISCUSSION
Despite over a century of research, there is no comprehensive account of visual apperceptive agnosia. This study evaluates the conjecture that central agnosic vision is like normal peripheral vision, and thus that the agnosic deficit is like crowding. The tests for object agnosia use complex displays that are susceptible to crowding. We measured the eccentricity-dependence of a standard observer’s performance of 14 tests commonly used for the diagnosis of visual agnosia, 10 of which were taken from two standard batteries, VOSP and BORB. For each test, our measurements on the standard observer assigned an equivalent eccentricity to each level of performance. Then, for each apperceptive agnosia patient, we used this mapping to convert each published performance score to its equivalent eccentricity. Equivalent eccentricity allows comparison of the deficit across all crowding-susceptible tasks. From the literature, we obtained the published scores on several standard agnosia tests by 10 individual patients and one group of PCA patients. For each patient, we found that all the crowding-susceptible tests yielded approximately the same equivalent eccentricity. Thus equivalent eccentricity was conserved across tests. This shows that agnosic is like eccentric vision.
Our results can be summarized by five findings: 1. a dichotomy between simple and complex displays, 2. the conservation across tasks of equivalent eccentricity, 3. conservation across tasks of crowding distance, 4. conservation, across eccentricity and agnosia, of the relative susceptibility of recognition of the many tests, and, 5. that crowding is not tightly linked to acuity.
1. Simple vs. complex displays. Agnosic is like eccentric vision, and the object-recognition deficit of agnosic patients is like peripheral crowding. Complex-display tasks are limited by crowding, and patients perform them poorly. Simple-display tasks are immune to crowding, and patients perform them well. In neurology clinics, acuity is usually tested with a simple one-letter display, which is immune to crowding, and is near normal in the patients.
2. Conservation, across tests, of equivalent eccentricity. Normally-sighted performance drops with eccentricity at a different rate for each task, so, for any poor score at a given task by a patient viewing directly, there is a larger equivalent eccentricity at which our normally-sighted observer would attain the same score. This becomes increasingly interesting when the patient has taken multiple tests, so our literature survey sought to find them all. Our key finding is that, when a patient’s scores on several tests are converted to equivalent eccentricities, they agree: Equivalent eccentricity is conserved across tasks. This is remarkable in light of the diversity of the tests and patients. Despite the obvious diversity of the tests (Table 2), they give the same equivalent eccentricity. The patients have diverse lesions, all accidental, which might be expected to produce diverse effects on different tests, too complicated to capture with any single parameter, yet equivalent eccentricity is enough. For any given patient, observer PMS viewing at a single eccentricity predicts the patient’s central performance of every complex-display test.
3. Conservation, across tests, of crowding distance. In normal eccentric vision, crowding distance is conserved across objects at each eccentricity (Pelli & Tillman, 2008). We have shown that one number, the apperceptive agnosia patient’s equivalent eccentricity, is enough to specify the patient’s ability to identify each of the ten diverse complex visual objects tested. Thus, across objects, each agnosia patient’s conservation of equivalent eccentricity implies that they also conserve crowding distance.
4. Conservation, from patients to eccentricity, of test susceptibility. Whether assessed across various degrees of agnosia or eccentricity, we find the same relative susceptibility of recognition of the ten objects for which we have data (Fig.7). If foveal agnosic vision is like eccentric vision, then one would expect this conservation of susceptibility. Alternatively, if agnosia and eccentricity limit vision in different ways then we would expect the diverse test objects to have different patterns of relative sensitivity for agnosia and eccentricity, contrary to what we found.
5. Crowding is not tightly linked to acuity. Peripheral identification of a complex display is usually crowding-limited, and thus independent of acuity. The complex displays used here to estimate equivalent eccentricity all use objects much bigger than the acuity size. Song, Levi, and Pelli (2014) report that anisometropic amblyopia patients have poor acuity and normal crowding, while our data suggest that another clinical condition (apperceptive agnosia) seems to greatly worsen crowding while sparing acuity. Combining their results with ours, Song et al. (2014) report a psychophysical double dissociation of acuity and crowding. We welcome further studies on these clinical populations to assess the suggested double dissociation and its neural correlates.
Crowding and apperceptive agnosia
We have shown that each apperceptive agnosia patient’s ability to identify diverse complex visual objects may be specified by one number, his or her equivalent eccentricity. This conservation of equivalent eccentricity, in each apperceptive agnosia patient, implies conservation of crowding distance
Crowding-like behavior in agnosia: Text.
When identification of cluttered or multi-part objects is impaired because of crowding, recognition can be restored by increasing the object size, increasing the spacing between the parts, or isolating the target part from the surrounding elements (Whitney & Levi, 2011; Levi, 2008; Martelli et al., 2005; Pelli et al., 2004). Crutch and Warrington (2007; 2009) reported two patients affected by posterior cortical atrophy (PCA) whose ability to recognize a central letter improved when the flanking distracters were farther away. In the case of a word, scaling the size of the text increases the letter spacing: This scaling reduces crowding and restores recognition. Similarly, HJA’s “reading is restricted to newspaper headlines or large print books” (Humphreys & Riddoch, 1987, p. 29). Buxbaum, Glosser, and Coslett (1999) report that “although W.B.’s visual acuity of 20/40 is adequate … he thought letter recognition to be less difficult with large stimuli”.
Crowding-like behavior in agnosia: Faces.
In the normal periphery, a facial feature is hard to identify when crowded by the other features, and isolating a part by removing the rest of the face or spreading the facial features apart restores recognition (Martelli et al., 2005). Similarly, HJA was much better at recognizing a facial feature presented alone than when presented in a face (Boutsen & Humphreys, 2002). HJA’s performance is unlike the well-known foveal face superiority effect (Tanaka & Farah, 1993; Tanaka & Sengco, 1997) and similar to the face inferiority effect due to crowding found in the normal periphery (Mäkelä, Näsänen, Rovamo, and Melmoth, 2001; Martelli et al., 2005). We imagine that an agnosia patient might occasionally see better by using his or her hand as a reduction tube to isolate a simple recognizable part of a face or street sign.
A glance at the neural substrates of crowding and apperceptive agnosia
Apperceptive agnosia may represent a defect in the ventral stream (Goodale et al., 1991; Goodale and Milner 1992), and is usually associated with either a bilateral or a right-unilateral occipito-temporal lesion that spares striate cortex and parietal areas (Humphreys, 1999). More recently it has been reported that a lesion in the left hemisphere near the VWFA (visual word form area) may lead to severe alexia and a mild prosopagnosia and, conversely, a lesion in the right hemisphere near the FFA (fusiform face area) may lead to prosopagnosia and a mild alexia (Behrmann & Plaut, 2014). Thus, object recognition deficits seem to be associated with distributed cortical networks (Berhmann & Plaut, 2013). Consistent with this view, recent fMRI studies found that a neural analog of visual crowding seems to be associated with a widespread network that involves all the early visual areas including the VWFA (Freeman, Donner, & Heeger, 2011; Anderson et al., 2012; Millin et al., 2013; Chen et al., 2014). Lesions may compromise this network in agnosia. In their study of crowding in 26 PCA patients, Yong et al. (2014) report a correlation between crowding and grey matter volume within the right collateral sulcus, between the fusiform and lingual gyri. Thus, crowding in the central vision of the agnosic patients may reflect limited plasticity in recovering from neural loss of the ventral stream, i.e. insufficient recruitment of other neurons to entirely make up for the loss. Our results speak only to the psychophysical behavior of agnosic and peripheral vision. Other studies are needed to identify the neural correlates. Even so, linking apperceptive agnosia and crowding as perceptual phenomena facilitates consideration of the computation underlying object recognition.
Conservation of number of neurons
Crowding field is the area enclosed by the crowding distance in every direction (also known as “combining field” and “integration field”, among other names). As eccentricity increases, the crowding distance (in deg at visual field) grows proportionally and the cortical magnification factor (mm/deg) drops inversely, so that their product, the crowding distance in mm at the cortex, is constant, independent of eccentricity, in all the cortical areas with logarithmic retinotopy: V1, V2, V3, V4/V8, LO1, and LO2 (Motter, 2007; Pelli, 2008). This implies a fixed cortical area within a crowding distance, i.e. crowding area is conserved across eccentricity. Since neural density (neurons per mm2 of cortical surface) is conserved across (normal) individuals, conservation of crowding area implies conservation of the number of cortical neurons in the crowding area (Rockel, Hiorns, & Powell, 1980; Braitenberg & Schüz, 1988; Pelli, 2008). Neural density is 0.12 1012 mm2 in most of the cortex and 0.31 1012 mm2 in V1 (Rockel et al., 1980). The cortical magnification scalar β varies slightly among visual areas (Larsson & Heeger, 2006). Thus the V1 crowding area of 2 × 12 mm2 contains 7.4 mm2 neurons, and the V2 (and V3 and hV4) crowding area of 1.6 × 10 mm2 contains 1.9 1012 neurons. (Relative to V2, the estimated area and count are 40% lower for LO1 and 40% higher for LO2.)
The site of crowding is still unknown.
Neurophysiology indicates that crowding may occur between V1 and V4/V8 (Freeman and Simoncelli, 2011; Freeman, Donner, & Heeger, 2011; Anderson et al., 2012; Millin et al., 2013; Chen et al., 2014; Harrison and Bex, 2015), and the conservation across eccentricity of the radial crowding distance in the logarithmically mapped areas makes V1, V2, V3, hV4, LO1, and LO2 likely candidates. All these areas conserve the number of neurons per crowding field across eccentricity. Let us suppose, for a moment, that one of these cortical areas is crowding-relevant, i.e. the site of crowding.
Across normal individuals, Vernier acuity is highly correlated with the cortical magnification factor in V1, and the threshold Vernier offset corresponds to a fixed distance in mm on the surface of V1 (Levi et al., 1985; Duncan & Boynton, 2003). Since acuity size and crowding distance both seem to be linked to cortical magnification, the Song et al. (2014) evidence for double dissociation of crowding and acuity suggests that acuity and crowding are linked to different areas. Acuity is tightly linked to V1, so crowding cannot be, but may be tightly linked to another cortical area.
Knowing that in normal vision there is a fixed number of neurons in a crowding field, independent of eccentricity, and that agnosic vision is like eccentric vision, we hypothesize that in agnosia as well, there is the same fixed number of neurons in a crowding field. We suppose that radial crowding distance, whether in agnosia after brain damage or in peripheral vision after normal development, is determined solely by the number of available neurons per square degree in the in the crowding-relevant cortical area, e.g. hV4, at the tested eccentricity. Confining ourselves to the crowding-relevant cortical area, once given the number of cortical neurons that fit in the area of a crowding field, then the neural density (per deg2) determines the extent of crowding. This neural density may be reduced by lower cortical magnification (in the periphery), take over by the other eye (in strabismic amblyopia), or cell death (in agnosia). This neural-density hypothesis would account for the known dependence of radial crowding distance on eccentricity and explain the new observation that loss of neurons in agnosia results in central vision that is like peripheral vision, limited by crowding. Note that this neural-density hypothesis merely extends the known conservation of number of neurons per crowding field (in the crowding-relevant cortical area) from normal to agnosic vision.
Crowding and object-category-specific deficits in visual agnosia
Though rare, visual agnosia has been studied with great interest for over a century in order to elucidate the basic mechanisms of object recognition. Part of the debate has focused on whether agnosia may occur as a domain-specific deficit, impairing some kinds of objects and tasks, while sparing others.
Neuropsychological studies of brain-damaged patients have found selective deficits for words (pure alexia), objects (pure visual object agnosia), and faces (prosopagnosia) (Farah 2004). However, when the deficit is selective, it usually turns out to be associative—not apperceptive—agnosia (Farah, 2004).
Conversely, patients with a pure apperceptive deficit are usually broadly impaired in the recognition of many categories of stimuli, not just one specific category. For instance, patients with pure alexia (cannot read words) are also impaired with digits and in discriminating black-and-white checkerboards (Starrfelt, Habekost & Leff, 2009; Mycroft, Behrmann & Kay, 2009). Patients with apperceptive object agnosia following a unilateral or bilateral lesion in the lateral occipital complex (LOC) seem to also be impaired with several kinds of stimuli (James et al., 2001; Ptak et al., 2014). Patients with apperceptive prosopagnosia may have trouble identifying other visually similar items like “Greebles” (Gauthier and Tarr, 1997). Gauthier, Behrmann, and Tarr (1999) suggested that object recognition tasks may be distributed in a multidimensional space defined by at least three relevant factors: expertise, categorization level, and stimulus class membership. Previous attempts to equate task difficulty were based on accuracy of normal observers. Gauthier et al. (1999) measured each prosopagnosia patient’s sensitivity and reaction time as a function of manipulations of the three factors. The authors found that prosopagnosia patients show a highly selective deficit for faces when performance is measured in terms of accuracy, but for non-face objects with increased categorization level (from subordinate to basic to exemplar) they have disproportionately lower sensitivity and higher reaction times. Similarly, in psychophysical testing, Starrfelt at al. (2010) found that the deficit of a patient with pure alexia (NN) was not restricted to letters, and NN’s central vision was like NN’s peripheral vision. In the authors’ words, this “could point to a form of foveal amblyopia, where shape perception is disproportionally impaired in the centre of the visual field.” (Starrfelt, Habekost, & Gerlach, 2010, page 253).
In short, the selectivity of the agnosic deficit has been debated, and part of the variation in performance across object categories may reflect task difficulty (but see also Riddoch et al. 2008 for a counterargument).
The absence of pure cases does not exclude the existence of domain-specific areas serving each category (Grill-Spector et al., 1998; Grill-Spector, Kushnir, Edelman, Itzchak & Malach, 1998; Murtha, Chertkow, Beauregard & Evans, 1999; Kourtzi & Kanwisher, 2000a; Doniger, Foxe, Murray, Higgins, Snodgrass & Schroeder, 2000; Grill-Spector et al., 2001 Andrews et al., 2010; Woohead et al., 2011). The presence of a category-selective module does not guarantee that pure deficits will occur in the clinical population. One possible reason for the dearth of pure cases is that lesions in these patients are seldom narrow enough to knock out just one category. It is also possible that each domain-specific area recruits a wider network in the occipito-temporal cortex, and the domain-specific networks overlap somewhat (Behrmann & Plaut, 2013).
The evidence for brain modules specific to particular kinds of object led us to expect that each patient would have diverse equivalent eccentricities, reflecting the kind of object most impaired by the lesion. Instead we find that each patient’s equivalent eccentricity is conserved across objects. It is remarkable that the plastic changes in recovery from brain damage converge on visual crowding that is so similar, across all complex image tasks, to normal peripheral retina. Perhaps each eye’s crowding distance is wholly determined by neural density in the crowding-relevant cortical area.
Agnosic is like eccentric vision
In general, the effect of crowding is strongly task- and stimulus-dependent. Here we showed that various tasks yield very different slopes of accuracy vs. eccentricity: from a mere −0.005 for two-letter identification to a whopping −0.1 for the similar-flanker condition. Converting a patient’s accuracy to equivalent eccentricity accounts for the way that crowding depends on task and stimulus. Similarly, the relative susceptibility of recognition of diverse objects to various eccentricities is conserved for various agnosias.
5. CONCLUSIONS
Conservation of each patient’s equivalent eccentricity.
We find that each apperceptive agnosia patient’s ability to identify diverse complex visual objects is specified by one number, his or her equivalent eccentricity. That is the eccentricity at which a standard observer’s peripheral vision is as poor as the patient’s central vision for that task. The conservation of equivalent eccentricity across tasks indicates that the recognition deficit in apperceptive agnosia is like visual crowding.
Our crowding hypothesis provides a one-parameter account of apperceptive agnosia that predicts performance of all the complex-object recognition tests. This enables succinct description of a phenomenon that historically has relied heavily on case studies of individual patients. The published patients included here have brain lesions of various sizes and locations, yet all conform to the equivalent-eccentricity model. To the extent that the findings reported here, based on 46 patients from 24 papers, are representative of all patients with apperceptive agnosia, it may be helpful to routinely convert raw test performance scores to equivalent eccentricities. Our crowding conjecture predicts conservation of equivalent eccentricity: Each patient’s equivalent eccentricity will be consistent across all complex-image tests. Table 2 provides a formula and parameter values to compute equivalent eccentricity from the performance score on 14 popular tests. Eccentricity-dependence varies hugely across tasks (Table 2) and very little across normally-sighted individuals.
Conservation of each test’s susceptibility.
Relative susceptibility of recognition in ten diverse visual tasks is conserved from testing with various eccentricities to testing with various agnosias. This recommends tabulating susceptibility, as in Table 2. We welcome extensions of this table to include more tests.
Clinical recommendation.
Thus, it may be helpful to explicitly test for crowding when characterizing the vision of agnosic patients. We recommend the Cambridge Crowding Cards (Atkinson et al., 1986; 1988), the Glasgow Acuity Cards (McGraw & Winn, 1993; sold by Keeler as the LogMAR Crowding Test), and the Pelli Clinical Test for Visual Crowding (Pelli et al., 2016), which are all designed to measure foveal crowding. However, any sensitive complex-display (with a high slope in Table 2) will do. We hope it will prove useful to routinely convert raw test scores with complex displays to equivalent eccentricity, to facilitate comparisons across tests and patients.
Neural density.
Finally, the neural-density hypothesis provides a parsimonious account of the surprising finding that agnosic is like eccentric vision. Perhaps both are limited by crowding and radial crowding distance is determined by neural density (per deg2) in the crowding-relevant cortical area.
ACKNOWLEDGMENTS
We thank Aenne Brielmann, Roberta Daini, Tomer Livne, Sarah Rosen, Lauren Vale, Carol Seaholm Volow, Xiuyun Wu, Angelica Zeller-Michaelson, and Pierluigi Zoccolotti for helpful comments. We particularly thank both J.A. Movshon and Reviewer 1 for suggesting that it would be worth looking at acuity as an alternate account. We thank PMS for his careful observations. This work was supported by: Italian Department of Health and Sapienza University (M.M.); Paola dei Mansi Fellowship (F.S.); NIH Grant R01-EY04432 (D.G.P.).
Footnotes
“Simultanagnosia” (Wolpert, 1924; Farah, 2004) is frequently associated with the Balint-Holmes syndrome (Balint, 1909/1995). Simultanagnosia patients have few signs of visual agnosia. Like apperceptive agnosic patients, their visual acuity is usually normal, and they fail to recognize a complex display as whole. However, unlike apperceptive agnosia patients, simultanagnosia patients do recognize single parts of a complex display, and have a complementary spectrum of symptoms, which may reflect the different computational functions of the dorsal visual areas (Mishkin, Ungerleider and Macko, 1983; Milner and Goodale, 1995). In the Navon local/global test, these patients tend to fail to recognize the global letter but succeed in recognizing the small local letter (Shalev et al., 2005; Mevorach et al., 2014). Simultanagnosia is associated with a deficit in the disengagement of attention from the objects (Farah, 1990; Coslett and Saffran, 1991), a general reduction in speed of visual processing (Luria, 1959; Balint, 1909/1995), and a deficit in combining space and object information (Coslett and Lie, 2008).
REFERENCES
- Adler A (1944). Disintegration and restoration of optic recognition in visual agnosia: Analysis of a case. Archives of Neurology and Psychiatry, 51, 243–259. [Google Scholar]
- Anderson EJ, Dakin SC, Schwarzkopf DS, Rees G, & Greenwood JA (2012). The neural correlates of crowding-induced changes in appearance. Current Biology, 22(13), 1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews TJ, Clarke A, Pell P, & Hartley T (2010) Selectivity for low-level features of objects in the human ventral stream. NeuroImage, 49, 703–711. [DOI] [PubMed] [Google Scholar]
- Astle AT, Blighe AJ, Webb BS, & McGraw PV (2014) The effect of aging on crowded letter recognition in the peripheral visual field. Invest Ophthalmol Vis Sci, 1;55(8), 5039–45. doi: 10.1167/iovs.14-14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Anker S, Evans C, Hall R, & Pimm-Smith E (1988). Visual acuity testing of young children with the Cambridge Crowding Cards at 3 and 6 m. Acta Ophthalmol (Copenh), 66(5), 505–508. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Pimm-Smith E, Evans C, Harding G, & Braddick O (1986). Visual crowding in young children. Doc Ophthalmol Proc Ser, 45, 201–213. [Google Scholar]
- Balint R (1909). Seelenlähmung des ‘‘Schauens’’, optische Ataxie, räumliche Störung der Aufmerksamkeit. Monattsschriften für Psychiatrie und Neurologie, 25, 51–81. (Translated in Cognitive Neuropsychology, 1995, 12, 265–281). [Google Scholar]
- Behrmann M & Kimchi R (2003). What does visual agnosia tell us about perceptual organization and its relationship to object perception? J Exp Psychol Hum Percept Perform 29(1), 19–42 [DOI] [PubMed] [Google Scholar]
- Behrmann M & Plaut DC (2013) Distributed circuits, not circumscribed centers, mediate visual recognition. Trends Cogn Sci 17(5), 210–9. [DOI] [PubMed] [Google Scholar]
- Behrmann M & Plaut DC (2014) Bilateral hemispheric processing of words and faces: evidence from word impairments in prosopagnosia and face impairments in pure alexia. Cereb Cortex 24(4), 1102–18. [DOI] [PubMed] [Google Scholar]
- Behrmann M & Williams P (2007) Impairments in part-whole representations of objects in two cases of integrative visual agnosia. Cogn. Neuropsychol 24(7), 701–30. [DOI] [PubMed] [Google Scholar]
- Behrmann M, & Nishimura M (2010). Agnosias. Wiley Interdisciplinary Reviews: Cognitive Science, 1(2), 203–213. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Moscovitch M, & Winocur G (1994). Intact visual imagery and impaired visual perception in a patient with visual agnosia. Journal of Experimental Psychology: Human Perception and Performance, 20(5), 1068. [DOI] [PubMed] [Google Scholar]
- Benson DF & Greenberg JP (1969). Visual form agnosia. Archives of Neurology, 20, 82–90. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Convento S, Banco E, Mattioli F, Tesio L, & Vallar G (2014). Improving ideomotor limb apraxia by electrical stimulation of the left posterior parietal cortex. Brain, awu343. [DOI] [PubMed]
- Boucart M, Moroni C, Despretz P, Pasquier F, & Fabre-Thorpe M (2010). Rapid categorization of faces and objects in a patient with impaired object recognition. Neurocase, 16(2), 157–168. [DOI] [PubMed] [Google Scholar]
- Bouma H (1970). Interaction effects in parafoveal letter recognition. Nature, 226 (241), 177–178. [DOI] [PubMed] [Google Scholar]
- Boutsen L, Humphreys GW (2002). Face context interferes with local part processing in a prosopagnosic patient. Neuropsychologia, 40, 2305–13. [DOI] [PubMed] [Google Scholar]
- Brainard DH (1997) The Psychophysics Toolbox, Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Braitenberg V & Schuüz A (1988). Cortex: Statistics and Geometry of Neuronal Connectivity edn 2. Heidelberg, Germany: Springer Verlag. [Google Scholar]
- Buxbaum L, Glosser G, & Coslett HB (1996). Relative Sparing of Object Recognition in Alexia-Prosopagnosia. Brain and Cognition, 32, 202–205. [Google Scholar]
- Buxbaum LJ, Glosser G, & Coslett HB (1999) Impaired face and word recognition without object agnosia. Neuropsychologia 37(1), 41–50. [DOI] [PubMed] [Google Scholar]
- Campion J & Latto R (1985). Apperceptive agnosia due to carbon monoxide poisoning: An interpretation based on critical band masking from disseminated lesions. Behavioral Brain Research, 15, 227–240. [DOI] [PubMed] [Google Scholar]
- Caffarra P, Vezzadini G, Dieci F, Zonato F, & Venneri A (2002). Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurological Sciences, 22(6), 443–447. [DOI] [PubMed] [Google Scholar]
- Chen J, He Y, Zhu Z, Zhou T, Peng Y, Zhang X, & Fang F (2014) Attention-dependent early cortical suppression contributes crowding. J Neurosci, 34, 10465–10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ST, Li RW, & Levi DM (2007). Crowding between first- and second-order letter stimuli in normal foveal and peripheral vision. Journal of Vision, 7(2), 10, 1–13, http://journalofvision.org/7/2/10/, doi: 10.1167/7.2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ST (2007) Learning to identify crowded letters: does it improve reading speed? Vision Res 47, 3150–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coslett H, & Lie G (2008). Simultanagnosia: when a rose is not red. J. Cogn. Neurosci 20, 36–48. [DOI] [PubMed] [Google Scholar]
- Coslett H, & Saffran E (1991). Simultanagnosia: to see but not two see. Brain, 114, 1523–1545. [DOI] [PubMed] [Google Scholar]
- Crutch S & Warrington E (2007). Foveal crowding in posterior cortical atrophy: A specific early-visual-processing deficit affecting word reading. Cognitive Neuropsychology, 24(8), 843–866. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, & Warrington EK (2009). The relationship between visual crowding and letter confusability: Towards an understanding of dyslexia in posterior cortical atrophy. Cognitive neuropsychology, 26(5), 471–498. [DOI] [PubMed] [Google Scholar]
- Crutch SJ (2014). Seeing why they cannot see: understanding the syndrome and causes of posterior cortical atrophy. Journal of neuropsychology, 8(2), 157–170. [DOI] [PubMed] [Google Scholar]
- Damasio AR (1990) Category-related recognition defects as a clue to the neural substrates of knowledge. Trends Neurosci 13(3), 95–8. [DOI] [PubMed] [Google Scholar]
- Daniel PM, & Whitteridge D (1961). The representation of the visual field on the cerebral cortex in monkeys. The Journal of Physiology, 159, 203–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Renzi E (1996). Le agnosie visive. In Denes G & Pizzamiglio L (Eds), Manuale di Neuropsicologia Cognitiva. Normalità e Patologia dei Processi Cognitivi Bologna: Zanichelli, p. 1426. [Google Scholar]
- Delvenne JF, Seron X, Coyette F, & Rossion B (2004) Evidence for perceptual deficits in associative visual (prosop)agnosia: a single-case study. Neuropsychologia, 42(5), 597–612. [DOI] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Snodgrass JG, Schroeder CE, & Javitt DC (2000). Activation timecourse of ventral visual stream object-recognition areas: high density electrical mapping of perceptual closure processes. Journal of Cognitive Neuroscience, 12(4), 615–621. [DOI] [PubMed] [Google Scholar]
- Duncan RO, & Boynton GM (2003). Cortical magnification within human primary visual cortex correlates with acuity thresholds. Neuron, 38(4), 659–671. [DOI] [PubMed] [Google Scholar]
- Efron R (1968). What is perception? In Cohen RS and Wartofky M (Eds), Boston studies in the philosophy of science New York: Humanities Press. [Google Scholar]
- Ehlers HE (1953). Clinical testing of visual acuity. AMA Archives of Ophthalmology, 49, 431–434. [DOI] [PubMed] [Google Scholar]
- Farah MJ (1990). Visual Agnosia: Disorders of Object Recognition and What they tell us about Normal Vision Boston, Massachusetts: The MIT Press. [Google Scholar]
- Farah MJ (2004). Visual Agnosia London, U.K.: The MIT Press. [Google Scholar]
- Farah MJ, Levinson KL, & Klein KL (1995) Face perception and within-category discrimination in prosopagnosia. Neuropsychologia 33(6), 661–74. [DOI] [PubMed] [Google Scholar]
- Fery P, & Morais J (2003). A case study of visual agnosia without perceptual processing or structural descriptions impairment. Cognitive neuropsychology, 20(7), 595–618. [DOI] [PubMed] [Google Scholar]
- Flom MC, Heath GG, & Takahaski E (1963). Contour interaction and visual resolution: Contralateral effects. Science, 142, 979–80. [DOI] [PubMed] [Google Scholar]
- Foulsham T, Barton JJ, Kingstone A, Dewhurst R, & Underwood G (2009) Fixation and saliency during search of natural scenes: the case of visual agnosia. Neuropsychologia 47(8–9), 1994–2003. [DOI] [PubMed] [Google Scholar]
- Freeman J, Donner TH, & Heeger DJ (2011). Inter-area correlations in the ventral visual pathway reflect feature integration. Journal of Vision, 11(4), 15, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell E, & Wilding J (2011). Development of a vocabulary of object shapes in a child with a very-early-acquired visual agnosia: A unique case. The Quarterly Journal of Experimental Psychology, 64(2), 261–282. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Behrmann M, & Tarr MJ (1999) Can face recognition really be dissociated from object recognition? J Cogn Neurosci 11(4), 349–70. [DOI] [PubMed] [Google Scholar]
- Gauthier I, & Tarr MJ (1997) Becoming a “Greeble” expert: exploring mechanisms for face recognition. Vision Res 37(12), 1673–82. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, & Gore JC (1999) Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nat Neurosci 2(6), 568–73. [DOI] [PubMed] [Google Scholar]
- Gilaie-Dotan S, Perry A, Bonneh Y, Malach R, & Bentin S (2009) Seeing with profoundly deactivated mid-level visual areas: non-hierarchical functioning in the human visual cortex. Cereb Cortex, 19(7), 1687–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovagnoli AR, Aresi A, Reati F, Riva A, Gobbo C, & Bizzi A (2009) The neuropsychological and neuroradiological correlates of slowly progressive visual agnosia. Neurol Sci, 30(2), 123–31. [DOI] [PubMed] [Google Scholar]
- Goodale MA & Milner AD (1992) Separate visual pathways for perception and action. Trends Neurosci,15(1), 20–5. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD, Jakobson LS, & Carey DP (1991) A neurological dissociation between perceiving objects and grasping them. Nature, 349, 154–156 [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, & Weintraub S (O. Segal, Illus) (1983). Boston Naming Test (2nd ed.). Philadelphia: Lea & Tebiger. [Google Scholar]
- Gordon N (1968). Visual agnosia in childhood VI: Preliminary communication, Developmental Medicine and Child Neurology, 10, 377–379. [DOI] [PubMed] [Google Scholar]
- Grainger J, Tydgat I, & Isselé J (2010). Crowding affects letters and symbols differently. Journal of Experimental Psychology: Human Perception and Performance, 36(3), 673. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Itzchak Y, & Malach R (1998) Cue-invariant activation in object-related areas of the human occipital lobe. Neuron, 21(1), 191–202. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H, Schütze C, Ebke M, & Spang K (2004). Differential impact of parvocellular and magnocellular pathways on visual impairment in apperceptive agnosia? Neurocase, 10(3), 207–214. [DOI] [PubMed] [Google Scholar]
- Hiraoka K, Suzuki K, Hirayama K, & Mori E (2009). Visual agnosia for line drawings and silhouettes without apparent impairment of real-object recognition: a case report. Behavioural neurology, 21(3), 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GW, & Riddoch MJ (1987). To see but not to see: A case study of visual agnosia Hillsdale, NJ: Erlbaum. [Google Scholar]
- Humphreys GW (1999) Integrative Agnosia. In Humphreys GW (Ed.) Case Studies in the Neuropsychology of Vision, (pp. 41–58), London: Psychology Press. [Google Scholar]
- Humphreys GW, Riddoch MJ & Quinlan PT (1985) Interactive processes in perceptual organization: Evidence from visual agnosia. In Posner MI & Marin OSM (Eds.), Attention & Performance XI Hillsdale, N. J.: Erlbaum. [Google Scholar]
- Irvine RS (1945). Amblyopia ex anopsia. Observations on retinal inhibition, scotoma, projection, light difference discrimination and visual acuity. Transactions of the American Ophthalmological Society, 66, 527–575. [PMC free article] [PubMed] [Google Scholar]
- James TW, Culham J, Humphrey GK, Milner AD, & Goodale MA (2003). Ventral occipital lesions impair object recognition but not object-directed grasping: an fMRI study. Brain, 126, 2463–2475. [DOI] [PubMed] [Google Scholar]
- Joubert S, Felician O, Barbeau E, Sontheimer A, Barton JJ, Ceccaldi M, & Poncet M, (2003). Impaired configurational processing in a case of progressive prosopagnosia associated with predominant right temporal lobe atrophy. Brain, 126(11), 2537–2550. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (1983). Boston Naming Test Philadelphia: Lea & Febiger. OCLC 10450471 [Google Scholar]
- Karnath HO, Rüter J, Mandler A, & Himmelbach M (2009). The anatomy of object recognition—visual form agnosia caused by medial occipitotemporal stroke. The Journal of Neuroscience, 29(18), 5854–5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiper DC, Zesiger P, Maeder P, Deonna T, & Innocenti GM (2002) Vision after early-onset lesions of the occipital cortex: I. Neuropsychological and psychophysical studies. Neural Plast 9(1), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte W (1923). Über die Gestaltauffassung im indirekten Sehen. Zeitschrift für Psychologie, 93, 17–82. [Google Scholar]
- Larsson J & Heeger DJ (2006). Two retinotopic visual areas in human lateral occipital cortex. J Neurosci, 26:13128–13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham K, & Whitaker D (1996) Relative roles of resolution and spatial interference in foveal and peripheral vision. Ophthalmic Physiol. Opt, 16, 49–57. [PubMed] [Google Scholar]
- Lê S, Cardebat D, Boulanouar K, Hénaff MA, Michel F, Milner D, Dijkerman C, Puel M, & Démonet JF (2002). Seeing, since childhood, without ventral stream: a behavioural study. Brain, 125(1), 58–74. [DOI] [PubMed] [Google Scholar]
- Leek CE, Patterson C, Paul MA, Rafal R, & Cristino F (2012) Eye movements during object recognition in visual agnosia. Neuropsychologia 50(9), 2142–53. [DOI] [PubMed] [Google Scholar]
- Legge GE, & Foley JM (1980). Contrast masking in human vision. Journal of the Optical Society of America, 70(12), 1458–1471. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Barnes J, Ridgway GR, Wattam-Bell J, Warrington EK, Fox NC, & Crutch SJ (2011). Basic visual function and cortical thickness patterns in posterior cortical atrophy. Cerebral cortex, 21(9), 2122–2132. [DOI] [PubMed] [Google Scholar]
- Lestou V, Lam JM, Humphreys K, Kourtzi Z, & Humphreys GW (2014) A dorsal visual route necessary for global form perception: evidence from neuropsychological fMRI. J Cogn Neurosci, 26(3):621–634. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, & Aitsebaomo AP (1985). Vernier acuity, crowding and cortical magnification. Vision research, 25(7), 963–977. [DOI] [PubMed] [Google Scholar]
- Levi DM, Song S, & Pelli DG (2007). Amblyopic reading is crowded. Journal of Vision, 7(2), 21, 1–17. [DOI] [PubMed] [Google Scholar]
- Levi DM (2008). Crowding - an essential bottleneck for object recognition: a mini-review. Vision Research, 48(5), 635–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissauer H (1890). Ein Fall von Seelenblindheit nebst einem Beiträge zur Theorie derselben [A case of visual agnosia with a contribution to theory]. Archiv für Psychiatrie, 21, 222–270. [Translated in Shallice, T., & Jackson, M. (1988). Lissauer on agnosia. Cognitive Neuropsychology, 5, 153–192.] [Google Scholar]
- Liu L, & Arditi A (2000). Apparent string shortening concomitant with letter crowding. Vision Research, 40(9), 1059–1067. [DOI] [PubMed] [Google Scholar]
- Loring DW, Sethi KD, Lee GP, & Meador KJ (1990). Neuropsychological performance in Hallervorden-Spatz syndrome: A report of two cases. Neuropsychology, 4(3), 191. [Google Scholar]
- Luria AR (1959). Disorders of “simultaneous perception” in a case of bilateral occipito-parietal brain injury. Brain, 82, 437–449. [DOI] [PubMed] [Google Scholar]
- Mäkelä P, Näsänen R, Rovamo J, & Melmoth D (2001). Identification of facial images in peripheral vision. Vision Research, 41(5), 599–610. [DOI] [PubMed] [Google Scholar]
- Mannan SK, Kennard C, & Husain M (2009). The role of visual salience in directing eye movements in visual object agnosia. Current biology, 19(6), R247–R248. [DOI] [PubMed] [Google Scholar]
- Martelli MM, Majaj NJ, & Pelli DG (2005). Are face processed like words? A diagnostic test for recognition by parts. Journal of Vision, 5, 58–70. [DOI] [PubMed] [Google Scholar]
- McGraw PV, & Winn B (1993). Glasgow acuity cards: a new test for the measurement of letter acuity in children. Ophthalmic and Physiological Optics, 13(4), 400–404. [DOI] [PubMed] [Google Scholar]
- McMonagle P, Deering F, Berliner Y, & Kertesz A (2006). The cognitive profile of posterior cortical atrophy. Neurology, 66, 331–8. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Shapira JS, & Clark DG (2007). “Apperceptive” alexia in posterior cortical atrophy. Cortex, 43(2), 264–70. [DOI] [PubMed] [Google Scholar]
- Metitieri T, Barba C, Pellacani S, Viggiano MP, & Guerrini R (2013). Making Memories: The Development of Long-Term Visual Knowledge in Children with Visual Agnosia. Neural plasticity, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevorach C, Shalev L, Green RJ, Chechlacz M, Riddoch MJ, & Humphreys GW (2014) Hierarchical processing in Balint’s syndrome: a failure of flexible top-down attention. Front Hum Neurosci, 27(8), 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millin R, Arman AC, Chung ST, & Tjan BS (2013). Visual crowding in V1. Cereb Cortex, Epub 10.1093/cercor/bht159 [DOI] [PMC free article] [PubMed]
- Milner AD, & Goodale MA (1995). The visual brain in action Oxford, UK: Oxford Press. [Google Scholar]
- Milner AD, Perrett DI, Johnston RS, Benson PJ, Jordan TR, Heeley DW, Bettucci D, Mortara F, Mutani R, Terrazzi E, & Davidson DLW (1991). Perception and action in “visual form agnosia”. Brain, 114, 405–428. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, & Macko KA (1983). Object vision and spatial vision: Two cortical pathways. Trends in Neurosciences, 6, 414–417. [Google Scholar]
- Motter BC & Simoni DA (2007). The roles of cortical image separation and size in active visual search performance. Journal of Vision, 7(2), 6–6. [DOI] [PubMed] [Google Scholar]
- Murtha S, Chertkow H, Beauregard M, & Evans A (1999) The neural substrate of picture naming. J Cogn Neurosci 11(4), 399–423. [DOI] [PubMed] [Google Scholar]
- Mycroft RH, Behrmann M, & Kay J (2009) Visuoperceptual deficits in letter-by-letter reading? Neuropsychologia, 47, 1733–1744. [DOI] [PubMed] [Google Scholar]
- Nandy AS, & Tjan BS (2007). The nature of letter crowding as revealed by first- and second-order classification images. Journal of Vision, 7(2), 5, 1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon D (1977) Forest before the trees. The precedence of global features in visual perception. Cognitive Psychology, 9(3), 353–383. [Google Scholar]
- Parkes L, Lund J, Angelucci A, Solomon JA & Morgan M (2001). Compulsory averaging of crowded orientation signals in human vision. Nature Neuroscience, 4 (7), 739–44. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Waugh SJ, Martelli M, Crutch SJ, Primativo S, Yong KX, Rhodes M, Yee K, Wu X, Famira HF, & Yiltiz H (2016) A clinical test for visual crowding. F1000Research 5:81. [Google Scholar]
- Pelli DG & Tillman KA (2008). The uncrowded window of object recognition. Nature Neuroscience, 11(10), 1129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG (1997) The VideoToolbox software for visual psychophysics: Transforming numbers into movies, Spatial Vision 10, 437–442. [PubMed] [Google Scholar]
- Pelli DG, Burns CW, Farell B, & Moore-Page DC (2006). Feature detection and letter identification. Vision research, 46(28), 4646–4674. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Majaj NJ, Raizman N, Christian CJ, Kim E, & Palomares MC (2009). Grouping in object recognition: The role of a Gestalt law in letter identification. Cognitive neuropsychology, 26(1), 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG, Palomares M & Majaj NJ (2004). Crowding is unlike ordinary masking: Distinguishing feature integration from detection. Journal of Vision, 4(12), 1136–1169. [DOI] [PubMed] [Google Scholar]
- Pelli DG (2008) Crowding: a cortical constraint on object recognition. Curr. Opin. Neurobiol 18, 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, & Humphreys GW (1995). Contrasting effects of letter-spacing in alexia: Further evidence that different strategies generate word length effects in reading. The Quarterly Journal of Experimental Psychology, 48(3), 573–597. [DOI] [PubMed] [Google Scholar]
- Ptak R, Lazeyras F, Di Pietro M, Schnider A & Simon SR (2014) Visual object agnosia is associated with a breakdown of object-selective responses in the lateral occipital cortex. Neuropsychologia, 60, 10–20. [DOI] [PubMed] [Google Scholar]
- Rey A (1941). L’examen psychologie dan les cas d’encéphalopathie traumatique (Les problèmes) [The psychological examination in cases of traumatic encephalopathy (Problems)]. Archives de Psychologie 28, 215–285. http://en.wikipedia.org/wiki/Rey-Osterrieth_Complex_Figure. Accessed 01.12.2016 [Google Scholar]
- Riddoch MJ, Johnston RA, Bracewell RM, Boutsen L, & Humphreys GW (2008). Are faces special? A case of pure prosopagnosia. Cognitive Neuropsychology, 25(1), 3–26. [DOI] [PubMed] [Google Scholar]
- Riddoch MJ & Humphreys GW (1987). A case of integrative visual agnosia. Brain, 110, 1431–1462. [DOI] [PubMed] [Google Scholar]
- Riddoch MJ & Humphreys GW (1993). Birmingham Object Recognition Battery (BORB) Hove, UK: Lawrence Erlbaum Associates. [Google Scholar]
- Rockel AJ, Hiorns RW, & Powell TP (1980). The basic uniformity in structure of the neocortex. Brain, 103, 221–244. [DOI] [PubMed] [Google Scholar]
- Rosen S, Chakravarthi R, & Pelli DG (2014) The Bouma law of crowding, revised: Critical spacing is equal across parts, not objects. Journal of Vision 14(6), 10, 1–15. http://www.journalofvision.org/content/14/6/10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S & Pelli DG (2014) A review of crowding and grouping suggests a unit for object recognition Manuscript submitted for publication.
- Sacks O (1998). The man who mistook his wife for a hat: And other clinical tales Simon and Schuster. [Google Scholar]
- Shalev L, Chajut E & Humphreys G,W (2005) Interactive perceptual and attentional limits in visual extinction. Neurocase, 11(6), 452–62. [DOI] [PubMed] [Google Scholar]
- Shelton PA, Bowers D, Duara R, & Heilman KM (1994). Apperceptive visual agnosia: A case study. Brain and Cognition, 25, 1–30 [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, & Vanderwart M (1980). A standardized set of 260 pictures: Norms for name agreement, familiarity and visual complexity. Journal of Experimental Psychology: Human Learning & Memory, 6, 174–215. [DOI] [PubMed] [Google Scholar]
- Song S, Levi DM, & Pelli DG (2014). A double dissociation of the acuity and crowding limits to letter identification, and the promise of improved visual screening. Journal of Vision, 14(5), 3. http://jov.arvojournals.org/article.aspx?articleid=2121640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrfelt R, Habekost T, & Leff AP (2009) Too little, too late: reduced visual span and speed characterize pure alexia. Cerebral Cortex 19(12), 2880–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrfelt R, Habekost T, & Gerlach C (2010). Visual processing in pure alexia: A case study. Cortex, 46(2), 242–255. [DOI] [PubMed] [Google Scholar]
- Sternberg S (2003) Process decomposition from double dissociation of subprocesses. Cortex 39, 180–182. [DOI] [PubMed] [Google Scholar]
- Stuart JA & Burian HM (1962). A study of separation difficulty: Its relationship to visual acuity in normal and amblyopic eyes. American Journal of Ophthalmology, 53, 471–477. [PubMed] [Google Scholar]
- Talairach J & Tournoux P (1988). Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging Thieme, New York. [Google Scholar]
- Tanaka JW & Farah MJ (1993). Parts and wholes in face recognition. Quarterly Journal of Experimental Psychology A, 46(2), 225–245. [DOI] [PubMed] [Google Scholar]
- Tanaka JW & Sengco JA (1997). Features and their configuration in face recognition. Memory and Cognition, 25(5), 583–592. [DOI] [PubMed] [Google Scholar]
- Thomas JP (1985). Effect of static-noise and grating masks on detection and identification of grating tar-gets. Journal of the Optical Society of America A, 2(9), 1586–1592. [DOI] [PubMed] [Google Scholar]
- Toet A & Levi DM (1992). The two-dimensional shape of spatial interaction zones in the parafovea. Vision Research, 32, 1349–1357. [DOI] [PubMed] [Google Scholar]
- Treisman AM & Gelade G (1980). A feature integration theory of attention. Cognitive Psychology, 12, 97–136. [DOI] [PubMed] [Google Scholar]
- Vallar G (2000). The methodological foundations of human neuropsychology: studies in brain-damaged patients. Handbook of neuropsychology, 1, 305–44. [Google Scholar]
- Vecera S & Behrmann M (1997). Spatial attention does not require preattentive grouping. Neuropsychology, 11, 30–43. [DOI] [PubMed] [Google Scholar]
- Warrington EK & Taylor AM (1973). The contribution of the right parietal lobe to object recognition. Cortex, 9, 152–164. [DOI] [PubMed] [Google Scholar]
- Warrington EK (1985). Agnosia: The impairment of object recognition. In Vinken PJ, Bruyn GW & Klawans HL (Eds.), Handbook of clinical neurology Amsterdam: Elsevier. [Google Scholar]
- Warrington EK & James M (1988). Visual apperceptive agnosia: A clinical-anatomical study of three cases. Cortex, 24, 13–32. [DOI] [PubMed] [Google Scholar]
- Warrington EK & James M (1991). Visual Object and Space Orientation Battery (VOSP) Thames Valley Test Company, Bury St Edmunds. [Google Scholar]
- Whitney D & Levi DM (2011) Visual crowding: a fundamental limit on conscious perception and object recognition, Trends in Cognitive Sciences, 15 (4), 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert I (1924) Die Simultanagnosie: Störung der Gesamtauffassung. Zeitschrift für di gesamte Neurologie und Psychiatrie, 93, 397–415. [Google Scholar]
- Woodhead ZV, Wise RJ, Sereno M, & Leech R (2011). Dissociation of sensitivity to spatial frequency in word and face preferential areas of the fusiform gyrus. Cereb Cortex, 21(10), 2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong KX, Shakespeare TJ, Cash D, Henley SM, Nicholas JM, Ridgway GR, Golden HL, Warrington EK, Carton AM, Kaski D, Schott JM, Warren JD, & Crutch SJ (2014). Prominent effects and neural correlates of visual crowding in a neurodegenerative disease population. Brain, 137, 3284–99. doi: 10.1093/brain/awu293. [DOI] [PMC free article] [PubMed] [Google Scholar]