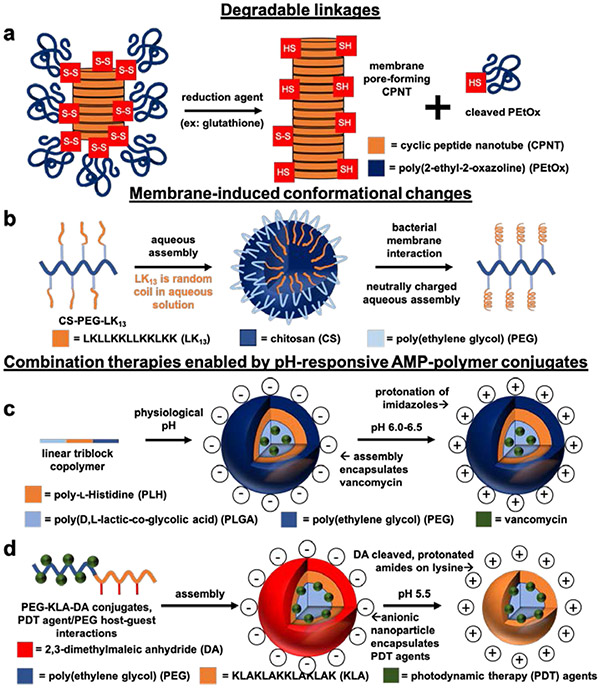
Figure 8.
Schematic illustrations of stimuli-responsive AMP-polymer conjugate systems. a) Redox-responsive poly(2-ethyl-2-oxazoline) (PEtOx)-cyclic peptide nanotube (CPNT) conjugates are activated by intracellular glutathione-triggered disulfide reduction, cleaving the PEtOx polymer to reveal bactericidal CPNTs.38 b) Conjugates of the AMP LK13 and PEGylated chitosan form nanospheres in aqueous environments. The neutral surface charge of the nanospheres allows for diffusion into the negatively charged extracellular matrix surrounding P. aeruginosa. Interaction with the bacterial membrane causes disassembly of the nanospheres and conversion of LK13 from random coil to a bactericidal α-helical conformation.36 c) Emulsion-templated fabrication of nanoparticles from pH-responsive linear triblock copolymers composed of poly(d,l-lactic-co-glycolic acid) (PLGA), poly(ethylene glycol) (PEG), and poly(l-histidine) (PLH) furnished pH-responsive nanoparticles encapsulating vancomycin. At physiological pH, the nanoparticles are anionic. Lowering the pH to 6.0-6.5 protonates the imidazole groups on PLH, rendering the nanoparticles cationic for increased interaction with bacterial membranes and subsequent vancomycin-induced bacteria killing.130 d) Capping lysine amines of the AMP KLA on PEG-KLA conjugates with pH-responsive 2,3-dimethylmaleic anhydride (DA) yields anionic conjugates at physiological pH. In acidic environments (pH 5.5), the DA caps are cleaved to reveal cationic lysines that target bacterial membranes. Encapsulation of PDT agents in the PEG-KLA conjugates enables light-triggered bacteria killing.131