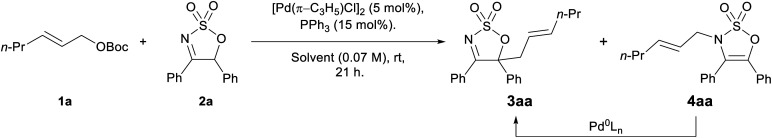
Selected optimisation results of the racemic Pd-AAA reactions of cyclic imine 2a with allyl carbonate 1a using triphenylphosphinea.
| |||
|---|---|---|---|
| Entry | Solvent | Yieldb (%) | |
| 3aa | 4aa | ||
| 1 | MeCN | 49c | 30c |
| 2d,e | MeCN | 77 | — |
| 3 | CH 2 Cl 2 | 70 | — |
| 4 | THF | 89 | — |
| 5 | PhMe | NR | |
| 6 | MeOH | 16 | 17 |
| 7 | DMF | 41 | 22 |
| 8 | DMSO | 61 | 12 |
Reaction conditions: 1a (0.2 mmol, 1.0 equiv.), 2a (0.22 mmol, 1.1 equiv.), [Pd(π-C3H5)Cl]2 (5 mol%), PPh3 (15 mol%), solvent (0.07 M w.r.t. 1a), rt, 21 h.
Yield determined by 1H NMR integration against an internal standard (1,2,3-trimethoxybenzene).
Isolated yield.
4aa was employed as the SM.
Reaction time: 17 h.
