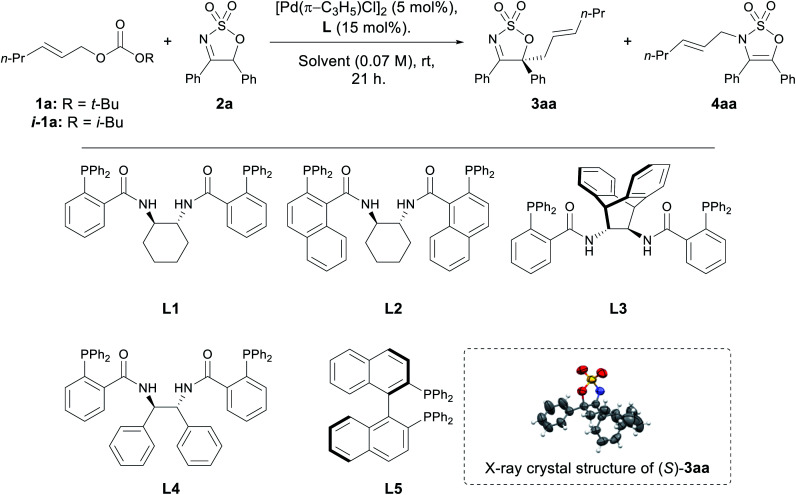
Selected optimisation of the Pd-AAA reactions of cyclic imine 2a with allyl carbonate 1a with chiral bidentate phosphine ligandsa.
| |||||
|---|---|---|---|---|---|
| Entry | Ligand | Solvent | Yieldb (%) | erc | |
| 3aa | 4aa | ||||
| 1d | L1 | THF | 90 | — | 93 : 7 |
| 2 | L2 | THF | NR | — | |
| 3 | L3 | THF | NR | — | |
| 4 | L4 | THF | 73 | 6 | 83 : 17 |
| 5 | L5 | THF | 97 | — | 91 : 9 |
| 6 e | L1 | CH 2 Cl 2 | 90 | — | 91 : 9 |
| 7 | L2 | CH2Cl2 | 17 | 10 | 84 : 16 |
| 8 | L3 | CH2Cl2 | 6 | 9 | 62 : 38 |
| 9 | L4 | CH2Cl2 | 54 | 6 | 85 : 15 |
| 10 | L5 | CH2Cl2 | 7 | — | 35 : 65f |
| 11e,g | L1 | CH2Cl2 | 78 | — | 91 : 9 |
| 12e,h | L1 | CH2Cl2 | 86 | — | 91 : 9 |
| 13e,i | L1 | CH2Cl2 | 84 | — | 92 : 8 |
| 14e,j | L1 | CH2Cl2 | 85 | — | 93 : 7 |
| 15e,k | L1 | CH2Cl2 | 61 | — | 91 : 9 |
Reaction conditions: 1a (0.2 mmol, 1.0 equiv.), 2a (0.22 mmol, 1.1 equiv.), [Pd(π-C3H5)Cl]2 (5 mol%), L (15 mol%), solvent (0.07 M w r t. 1a), rt, 21 h.
Yield determined by 1H NMR integration against an internal standard (dimethyl sulfone or trans-stilbene oxide).
Enantiomeric ratio determined by chiral HPLC.
Reaction reached completion after 3 h.
Reaction reached completion after 1 h.
Reversed enantioselectivity compared to entry 5.
[Pd(π-C3H5)Cl]2 (2.5 mol%), L (7.5 mol%).
Reaction concentration halved (0.04 M w. r. t. 1a).
i-1a (1.0 equiv.) used instead of 1a.
Pd2dba3·CHCl3 (5 mol%).
4aa was employed as SM.
