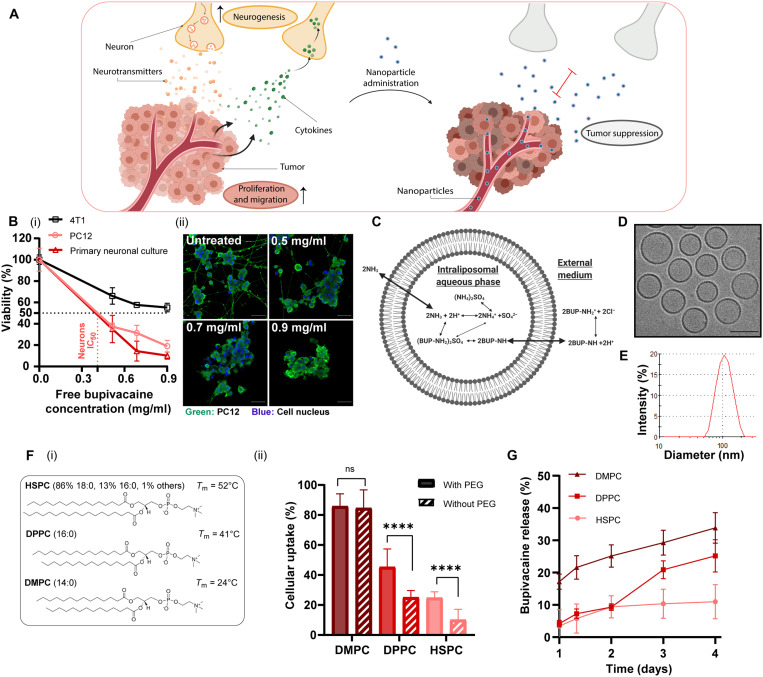
Fig. 1. Analgesic nanoparticles as a tool for treating neurons within breast cancer tumors.
Cross-talk between cancer cells and nerves supports cancer cell proliferation and migration; reciprocally, neurite growth is promoted through secretion of cytokines by cancer cells. We used lipid nanoparticles, liposomes, loaded with bupivacaine (L-BUP) to curb nerve/cancer cross-talk and inhibit tumor growth (A). Viability of primary neurons, PC12, and triple-negative breast cancer cells (4T1) after treatment with bupivacaine [(B), i] (normalized to the untreated group) and confocal imaging [(B), ii] (scale bars, 50 μm). Liposomes (100 nm) composed of HSPC (hydrogenated soybean phosphatidylcholine), cholesterol, and DSPE–PEG-2000 (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy–polyethylene glycol 2000; 55:40:5 molar ratio) were loaded with bupivacaine using ammonium sulfate gradient (C) and characterized by cryogenic transmission electron microscopy (cryo-TEM) (D) (scale bar, 100 nm) and dynamic light scattering (E) (PDI < 0.1). Various lipid formulations were compared for their neuronal uptake: HSPC, DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine), and DMPC (2-dimyristoyl-sn-glycero-3-phosphocholine), with cholesterol and with/without DSPE–PEG-2000 [(F), i]. PC12 cells were incubated with rhodamine-labeled liposomes and analyzed for their cellular uptake [(F), ii]. L-BUP release profile was conducted at 37°C, comparing the stability of the different formulations (G). Results are presented as means ± SD (between two and three independent repetitions performed in at least three replicates). Two-way analysis of variance (ANOVA) was used for statistical analysis of [(B), i] and one-way ANOVA for [(F), ii, and (G)] with multiple comparisons test adjusted P value; ****P < 0.0001. IC50, median inhibitory concentration; ns, not significant.