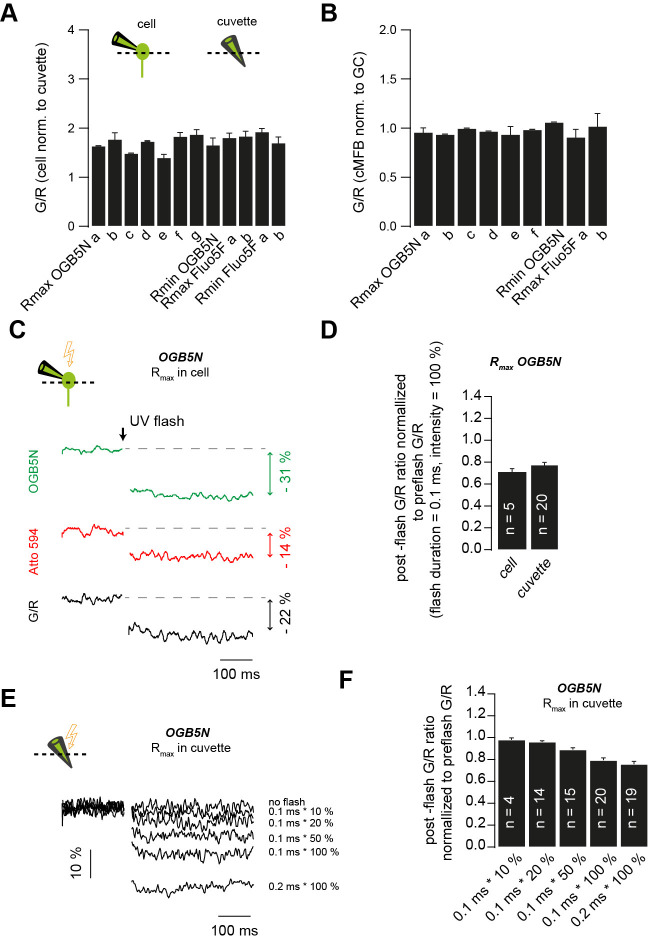
Figure 3. Ca2+ uncaging dose-response curve measured with deconvolution of EPSCs.
(A) Illustration of the cellular connectivity in the cerebellar cortex showing the pre- and postsynaptic compartments during paired whole-cell patch-clamp recordings and Ca2+ uncaging with UV-illumination. (B) Two-photon microscopic image of a cMFB and a GC in the paired whole-cell patch-clamp configuration. (C) Three different recordings showing UV-flash evoked EPSC (top trace) and cumulative release rate measured by deconvolution analysis of the EPSCs (bottom trace). The peak Ca2+ concentration, quantified with two-photon Ca2+ imaging, is indicated in each panel. represents the time constant from mono-exponential fit, represents the time constant of the fast component of bi-exponential fit. Note the different lengths of the baselines in the three recordings. (D) Plot of release rate versus post-flash Ca2+ concentration. Gray open circles represent data from capacitance measurements (Figure 2) and black triangles represent data from deconvolution analysis of EPSC (n = 57 recordings obtained from 42 paired cells). Gray and black lines represent fits with a Hill equation of the capacitance (as shown in Figure 1F) and the deconvolution data, respectively. The best-fit parameters for the fit on the deconvolution data were Vmax = 6*107 ms−1, KD = 7.6*105 µM, and n = 1.6. Red, blue, and brown symbols correspond to the traces in (C). (E) Plot of synaptic delay versus post-flash Ca2+ concentration (n = 59 recordings obtained from 43 paired cells). Note that two recordings was removed from the analysis because the exponential fit led to a negative value of the delay Gray open circles represent data from capacitance measurements, and black triangles represent data from deconvolution analysis of EPSC. Red, blue, and brown symbols correspond to the traces in (C).
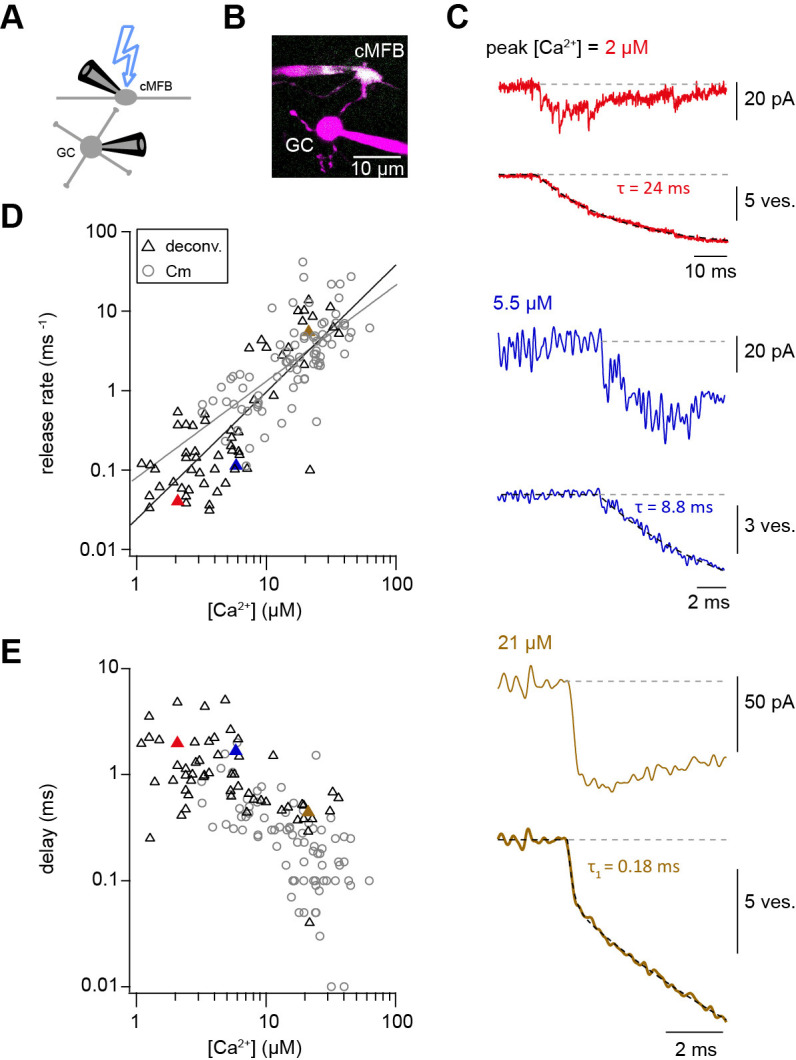
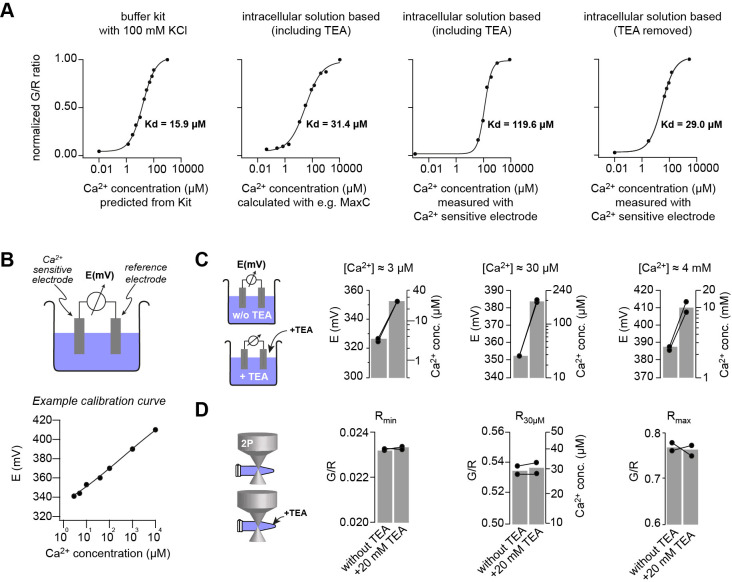
Figure 3—figure supplement 1. Measuring the KD of the Ca2+ sensitive dyes.
Figure 3—figure supplement 2. Comparison of brief versus long UV illumination to rule out fast Ca2+ overshoots.
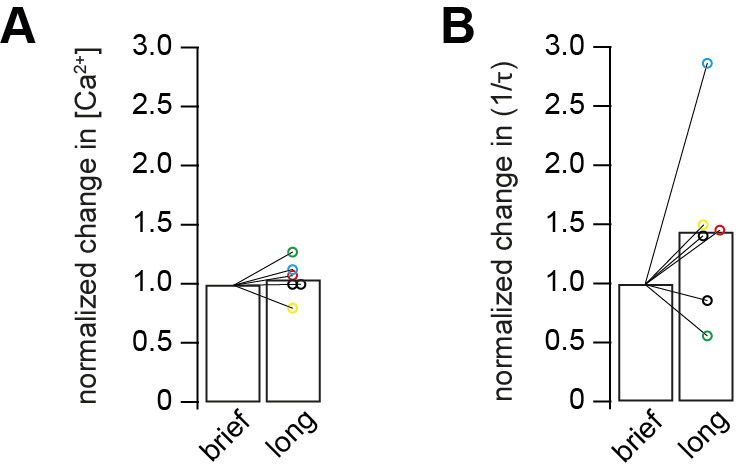
Figure 3—figure supplement 3. Correction for the post-flash changes in the fluorescent properties of the intracellular solution.
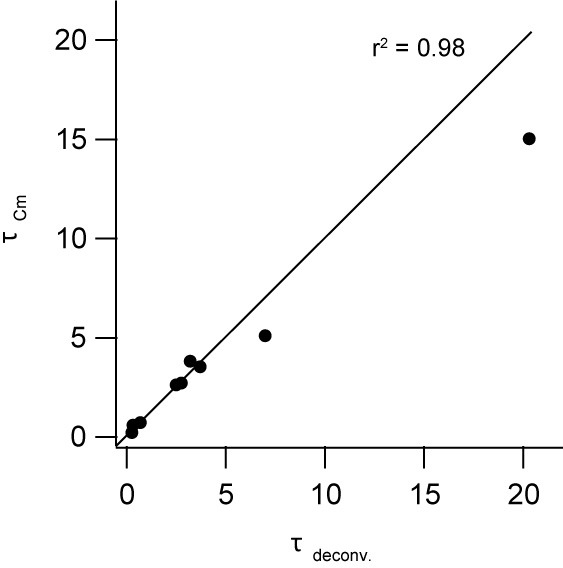
Figure 3—figure supplement 4. Comparison of the time constants obtained from presynaptic capacitance measurements (τCm) and analysis of postsynaptic current recordings (τdeconv).