Fig. 3.
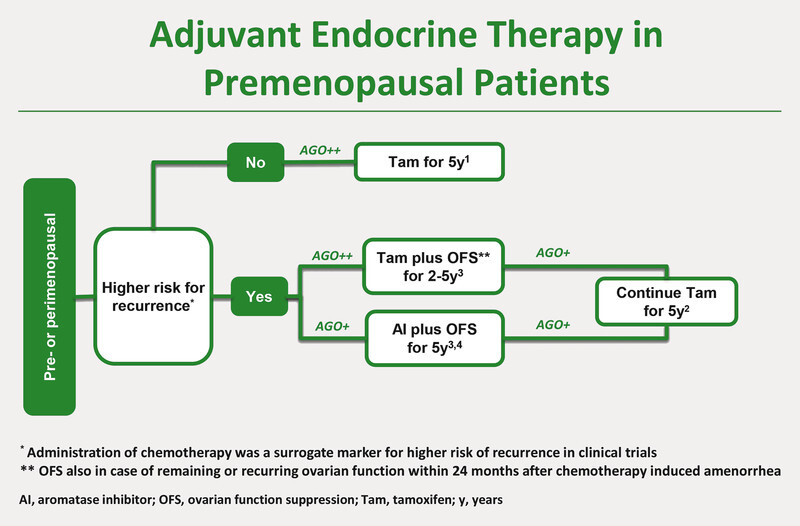
Algorithm Adjuvant Endocrine Therapy in Premenopausal Patients. 1 Early Breast Cancer Trialistsʼ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011; 378: 771 – 784. 2 Davies C, Pan H, Godwin J et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381: 805 – 806. 3 Goss PE, Ingle JN, Martino S et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005; 97: 1262 – 1271. 4 Francis PA, Regan MM, Fleming GF et al. The SOFT Investigators and the International Breast Cancer Study Group. Adjuvant Ovarian Suppression in Premenopausal Breast Cancer. N Engl J Med 2015; 372: 436 – 446. 5 Pagani O, Regan MM, Walley BA et al. TEXT and SOFT Investigators; International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 2014; 371: 107 – 118.
