Fig. 8.
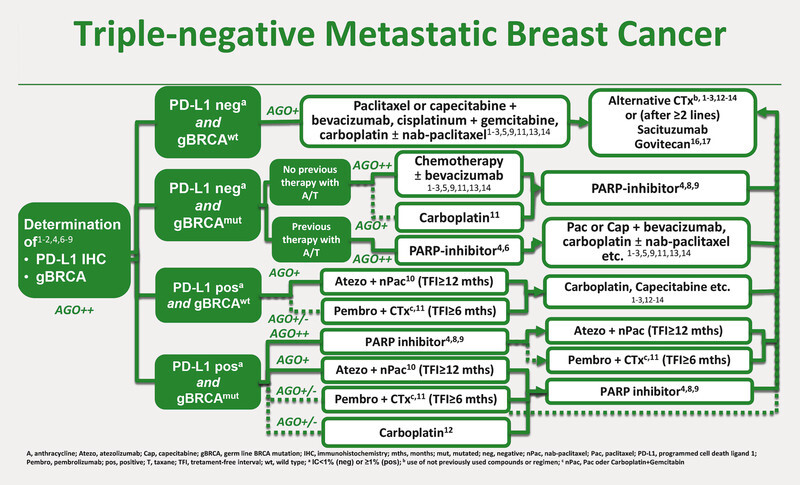
Algorithm Triple-negative Metastatic Breast Cancer. 1 Cardoso F, Senkus E, Costa A et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC4). Ann Oncol 2018; 29: 1634 – 1657. 2 Condorelli R, Mosele F, Verret B et al. Genomic alterations breast cancer: level of evidence for actionability according to ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol 2019; 30: 365 – 373. 3 Hu XC, Zhang J, Xu BH et al. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2015; 16: 436 – 446. 4 Litton JK, Rugo HS, Ettl J et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018; 379: 753 – 763. 5 Miles DW, Diéras V, Cortés J et al. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann Oncol 2013; 24: 2773 – 2780. 6 Miller K, Wang M, Gralow J et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007; 357: 2666 – 2676. 7 Miller KD, Chap LI, Holmes FA et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 2005; 23: 792 – 799. 8 Robson M, Im S-A, Senkus E et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 2017; 377: 523 – 533. 9 Robson M, Tung N, Conte P et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physicianʼs choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol 2019; 30: 558 – 566. 10 Schmid P, Adams S, Rugo HS et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 2018; 379: 2108 – 2121. 11 Cortes J, Cescon DW, Rugo HS et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020; 396: 1817 – 1828. 12 Tutt A, Tovey H, Cheang MCU et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med 2018; 24: 628 – 637. 13 Twelves C, Cortes J, Vahdat L et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies [published correction appears in Breast Cancer Res Treat 2015; 149: 313. Breast Cancer Res Treat 2014; 148: 553 – 561. 14 Yardley DA, Coleman R, Conte P et al. nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: results from the tnAcity trial. Ann Oncol 2018; 29: 1763 – 1770. 15 Zielinski C, Láng I, Inbar M et al., TURANDOT investigators. Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first line treatment for HER2-negative metastatic breast cancer (TURANDOT): primary endpoint results of a randomised, open-label, noninferiority, phase 3 trial. Lancet Oncol 2016; 17: 1230 – 1239. 16 Bardia A, Hurvitz SA, Tolaney SM et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med 2021; 384: 1529 – 1541. 17 Bardia A, Mayer IA, Vahdat LT et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med 2019; 380: 741 – 751
