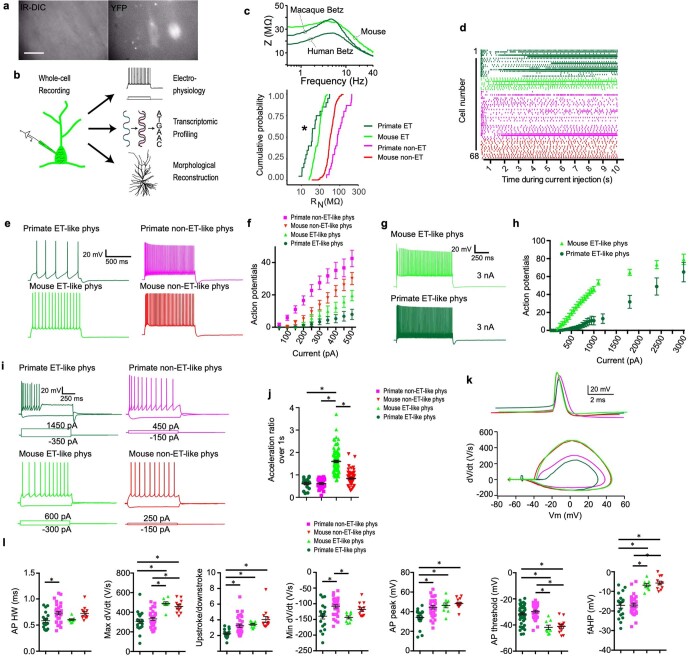
Extended Data Fig. 12. Differences in spike trains produced by L5 glutamatergic neurons and single spike properties across species.
a, Example IR-DIC (left) and fluorescence (right) images obtained from a macaque organotypic slice culture. Note the inability to visualize the fluorescently labelled neurons in IR-DIC because of dense myelination. All human and macaque recordings were from labelled neurons. Scale bar, 50 μm. b, Patch–seq involves the collection of morphological, physiological and transcriptomic data from the same neuron. Following electrophysiological recording and cell filling with biocytin via whole-cell patch clamp, the contents of the cell are aspirated and processed for RNA sequencing. This permits a transcriptomic cell type to be pinned to the physiologically probed neuron. c, Top, example ZAP profiles for the neurons shown in Fig. 6f–h. Bottom, cumulative probability distribution showing input resistance for physiologically defined L5 neuron types from primates versus mice. *P = 0.0064, Kolmogorov–Smirnov test between mouse and primate extratelencephalic neurons. d, Raster plot of spike times during 1-s epochs of a 10-s injection of DC current, with colour coding as in c. Primate extratelencephalic neurons (pooled data from humans and macaques, n = 20) displayed a distinctive decrease followed by a pronounced increase in firing rate over the course of the current injection, whereas other neuron types did not (primate intratelencephalic neurons, n = 30; mouse extratelencephalic neurons, n = 8; mouse intratelencephalic neurons, n = 12). Notably, a similar biphasic-firing pattern is observed in macaque corticospinal neurons in vivo during prolonged motor movements95,96, suggesting that the firing pattern of these neurons during behaviour is intimately tied to their intrinsic membrane properties. The acceleration in spike times of rodent extratelencephalic neurons has been attributed to the expression of Kv1-containing voltage-gated K+ channels, encoded by genes such as the conserved extratelencephalic gene KCNA1 (ref.41). e, Example voltage responses to a 1-s, 500-pA current injection. f, Action potentials (mean ± s.e.m.) as a function of the amplitude of injected current. Primate extratelencephalic neurons display the shallowest relationship between action potential and injected current, perhaps partially because of their exceptionally low input resistance (primate extratelencephalic neurons, n = 20; primate intratelencephalic neurons, n = 30; mouse extratelencephalic neurons, n = 9; mouse intratelencephalic neurons, n = 12). g, Voltage responses to a current injection with a 1-s, 3-nA step. h, Action potentials (mean ± s.e.m.) as a function of injected current for a subset of experiments in which the amplitude of injected current was increased incrementally to 3 nA. Although both mouse (n = 9) and primate (n = 10) extratelencephalic neurons could sustain high firing rates, primate neurons required 3 nA of current over 1 s to reach similar average firing rates as mouse extratelencephalic neurons. i, Example voltage responses to current injections with 1-s depolarizing steps. The amplitude of the current injection was adjusted to produce roughly ten spikes. Also shown are voltage responses to a hyperpolarizing current injection. j, The firing rate (mean ± s.e.m.) of primate extratelencephalic (n = 18), primate intratelencephalic (n = 30) and mouse intratelencephalic (n = 86) neurons decreased during the 1-s step current injection, whereas the firing rate of mouse extratelencephalic neurons increased (n = 110). The acceleration ratio is the ratio of the second to the last interspike interval. *P < 0.05, Bonferroni-corrected two-sided t-test. k, Example single action potentials (above) and phase plane plots (below). l, Various features of action potentials (mean ± s.e.m.) are plotted as a function of cell type (primate extratelencphalic, n = 20; primate intratelencephalic, n = 30; mouse extratelencephalic, n = 9; mouse intratelencephalic, n = 12). Notably, action potentials in primate extratelencephalic neurons were reminiscent of fast spiking interneurons, in that they were shorter and more symmetrical compared with action potentials in other neuron types/species. Intriguingly, the K+-channel subunits Kv3.1 and Kv3.2, which are implicated in fast-spiking physiology97, are encoded by highly expressed genes (KCNC1 and KCNC2) in primate extratelencephalic neurons (Fig. 6c). *P < 0.05, Bonferroni-corrected two-sided t-test.