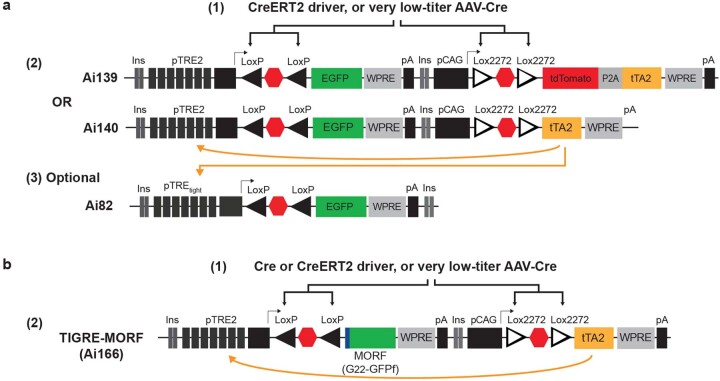
Extended Data Fig. 1. Genetic strategy for sparse, robust and consistent brain-wide neuronal labeling.
a, Schematic diagram showing the first approach of sparse and robust labeling, involving the combination of CreERT2 transgenic driver line or Cre-expressing AAV (1) with the GFP-expressing TIGRE2.0 reporter line Ai139 or Ai140 (2). Very low dose tamoxifen induction of CreERT2 (Supplementary Table 1) or very low-titer AAV-Cre delivery results in activation of the reporter in a spatially sparse manner. Transgenic reporter expression of GFP is robust and consistent across different cells. An optional addition is to cross in the GFP-expressing TIGRE1.0 reporter line Ai82 (3), so that the tTA2 from Ai139 or Ai140 will activate the expression of GFP from two alleles – Ai139/Ai140 and Ai82, further increasing the level of GFP within Cre+ cells. b, Schematic diagram showing the second approach of sparse and robust labeling, involving the combination of Cre or CreERT2 transgenic driver line or Cre-expressing AAV (1) with the GFP-expressing sparse reporter line TIGRE-MORF (Ai166) (2). In TIGRE-MORF (Ai166), the GFPf transgene is not translated at baseline due to the out-of-frame G22 repeat relative to the open reading frame of GFPf, which lacks its own translation start codon. During DNA replication or repair, rare events of stochastic frameshift of the mononucleotide repeat result in correction of the translation frame (i.e., G22 to G21) and produce expression of the GFPf protein in a small subset of cells. Ai166 exhibits a labeling frequency of 1-5% when crossed to different Cre driver mouse lines26. Even with this frequency, we find that combining Ai166 with many Cre driver lines densely expressing the Cre transgene does not produce sufficient sparsity to readily untangle the axonal ramifications, whereas combining it with Cre lines that are already relatively sparse, or with CreERT2 lines with intermediate dosing level of tamoxifen (Supplementary Table 1), results in very sparse labeling. The use of membrane associated GFPf also enables robust labeling of very thin axon fibers. Leaky background expression of GFP reported in other TIGRE2.0 lines24 is not present in Ai166 mice due to the strict dependency of translational frameshift for the expression of GFPf reporter, making Ai166 an ideal reporter line for sparse and strong labeling of various neuronal types across the brain. Our labeling strategy using stable and universal transgenic reporter mouse lines coupled with a variety of sparse Cre delivery methods has several advantages. First, the TIGRE2.0-based transgenic reporter lines, especially Ai166 which expresses a farnesylated GFP, produce very bright GFP labeling of axon fibers under fMOST imaging, revealing numerous terminal boutons, an essential requirement for obtaining truly complete morphologies. Second, this strategy enables sparse labeling across multiple regions within the same brain, improving efficiency compared to other methods (e.g., in vivo electroporation or stereotaxic virus injection). Third, the labeling is highly consistent from cell to cell, cell type to cell type, region to region, and brain to brain, reducing variability and enhancing reproducibility. Finally, sparse Cre recombination can be achieved through the use of transgenic Cre or CreERT2 driver lines labeling any neuronal type, or low-dose Cre viral vectors delivered through either local or systemic (e.g., retroorbital) injections.