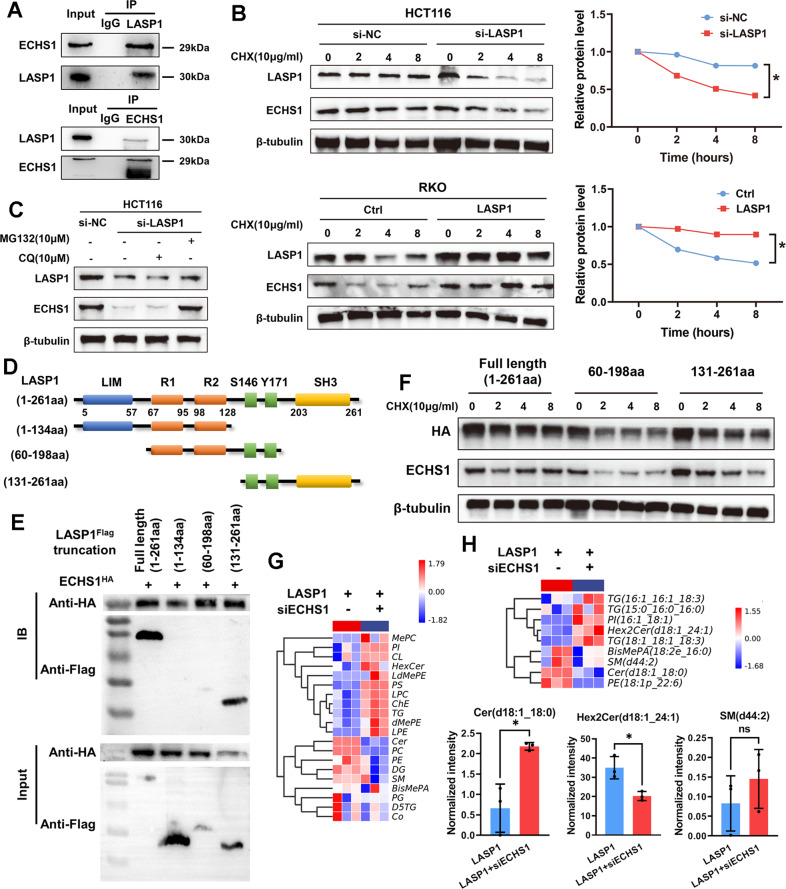
Fig. 2. ECHS1 is essential for LASP1-mediated sphingomyelin-metabolism imbalance by interacting with SH3 domain of LASP1 in CRC.
A Immunoprecipitation-assay analysis of endogenous interaction between ECHS1 and LASP1 in SW480 cells. B Western blot assay of LASP1 and ECHS1 protein and quantification of ECHS1 relative level at the indicated time in HCT116 cells transfected with siLASP1 or siNC (upper figure) or RKO cells transfected with LV-LASP1 or LV-Ctrl (lower figure) after CHX treatment to block protein synthesis. C Western blot assay of the level of LASP1 and ECHS1 protein with application of chloroquine (10uM) or MG132 (10uM) after 8 h. D Schematic presentation of LASP1 structure features. The structures of truncated LASP1 are listed behind those of the full-length proteins. E Western blotting results indicate the binding domain of ECHS1 and LASP1 in 293 T cells. F Western blot assay of HA and ECHS1 protein in 293 T cells transfected with plasmid HA-LASP1 full length (1–261aa), HA-LASP1 (60–199aa), or HA-LASP1(131–261aa) after CHX treatment to block protein synthesis. G Heatmap of the LC–MS assay for RKO-LASP1 and RKO-LASP1 transfected with siECHS1 cells. H Heatmap and histogram of depicting the differentially expressed lipomics for RKO-LASP1 and RKO-LASP1 plus siRNA-ECHS1 cells.