Abstract
Tolvaptan (TLV) is a vasopressin V2 receptor antagonist that increases free water excretion. However, there are few reports of the use of TLV in pediatric patients with nephrotic syndrome (NS). The efficacy of TLV for the management of edema and hyponatremia in a 12-year-old boy with refractory NS is demonstrated. In this patient, refractory NS developed at 5 years of age, and remission was maintained with several immunosuppressive agents. He was admitted to hospital due to relapse with oliguria, edema, hypoalbuminemia, and hyponatremia. Infusion of human albumin and furosemide did not increase his urine volume, and hyponatremia worsened. Administration of TLV increased urine volume and improved his edema and hyponatremia. There were no adverse effects except for slow elevation of the serum sodium level. Serum osmolality increased gradually, and urine osmolality remained at low levels during TLV treatment. Additionally, a decrease in the sum of the urinary sodium and potassium concentrations was useful to predict the response to TLV and to assess the therapeutic effect of TLV as a biomarker for monitoring. TLV is effective for the management of fluid and electrolyte balance in pediatric NS patients. Early administration of TLV is considered a useful therapy for hyponatremia and refractory edema resistant to other diuretics.
Keywords: Tolvaptan, Vasopressin type 2 receptor antagonist, Edema, Hyponatremia, Nephrotic syndrome
Introduction
The selective vasopressin V2 receptor antagonist tolvaptan (TLV) is a relatively new diuretic with a unique mechanism of action, which stimulates elimination of electrolyte-free water [1]. Although TLV has shown evidence of effectiveness in patients with heart failure and hepatic edema [2, 3], there are few reports of its use in pediatric patients with nephrotic syndrome (NS). We report a refractory pediatric NS case with edema and hyponatremia successfully treated with TLV.
Case report
A 12-year-old boy was diagnosed with idiopathic NS (steroid-resistant) at the age of 5 years. The first renal biopsy at the age of 5 years showed minimal change. He had first remission with various immunosuppressant medications (cyclosporine, cyclophosphamide, mizoribine) after admission for 6 months; however, he developed recurrence frequently after the first remission. Remission was maintained for more than 2 years after mycophenolate mofetil (MMF) was added at the age of 7 years. However, he had multiple relapses after withdrawal of MMF at the age of 11 years.
At the age of 12 years and 5 months, he had a relapse associated with gastroenteritis, and he achieved remission after 3 cycles of methylprednisolone pulse therapy and oral tacrolimus (Tac). Administration of MMF was resumed after remission was achieved; however, proteinuria returned after only 3 weeks of remission. 4 days later, he had an episode of fever with influenza type B, and he was admitted to the hospital because of oliguria and severe edema.
On admission, he had a 2.9-kg weight gain, fever with a temperature of 37.8 °C, and tachycardia. Furthermore, physical examination showed a poor general condition, unrelenting nausea, and lower leg edema. Urine examination showed massive proteinuria, with a morning urine protein of 9016 mg/dL, ratio of urine protein to urine creatinine (UP/Cr) of 80.2 g/gCr, urinary specific gravity of 1.050, urinary sodium of 71.0 mEq/L, fractional excretion of sodium (FENa) of 0.21%, and the sum of urinary sodium and potassium concentrations (U–Na + K) of 139.5 mEq/L.
Blood examination showed a hemoglobin concentration of 16.8 g/dL, indicating intravascular volume depletion, and a decreased serum albumin level at 2.1 g/dL. It also showed decreased serum sodium (129 mEq/L) and serum osmolality (264 mOsm/L). Furthermore, the cardiothoracic ratio (CTR) on chest X-ray was 45.0%, and ultrasonography showed collapse of the inferior vena cava.
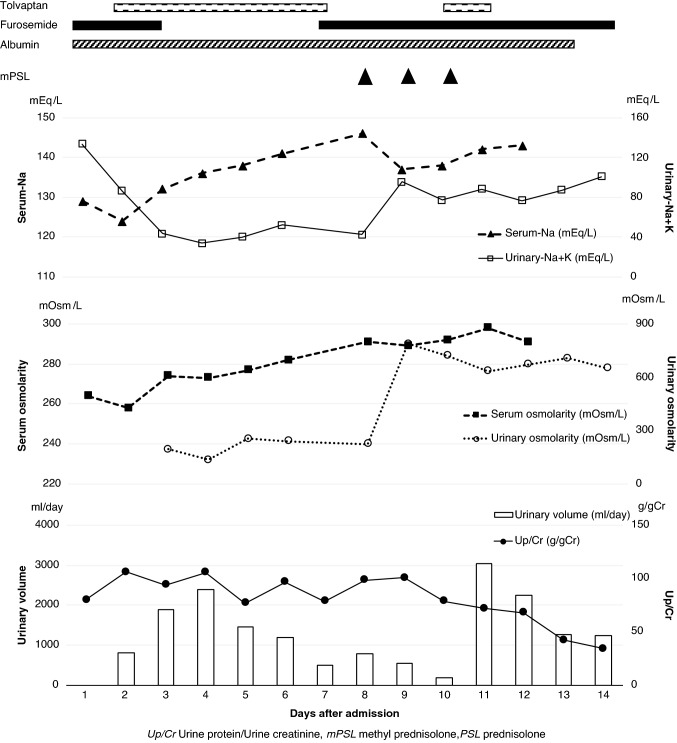
After his admission, MMF was discontinued due to the influenza infection, and intravenous fluids and an albumin preparation were administered to improve volume depletion. However, hypoalbuminemia and hyponatremia deteriorated further (Alb 2.1–0.8 g/dL, Na 129–124 mEq/L), and he had impaired renal function (serum creatinine 0.5–0.78 mg/dL) on the day after admission. Although he was given an albumin preparation and furosemide after sodium correction with hypertonic saline infusion, oliguria and severe edema persisted. After adding TLV 7.5 mg/day, urine volume increased significantly, and the serum sodium level increased gradually. Excretion of free water was demonstrated by the fact that U–Na + K decreased in step with starting TLV (Fig. 1). Furthermore, serum osmolality (S-OSM) increased gradually after administering TLV although there was hypoosmolality on admission. Urine osmolality (U-OSM) was lower than S-OSM after TLV was given. In the course of acute-phase treatment, although the CTR of the chest X-ray changed to show cardiac enlargement, and ultrasonographic observation showed expansion of the inferior vena cava, these findings improved with the administration of TLV. His edema improved and body weight decreased about 3.8 kg after TLV was administered.
Fig. 1.
Clinical course of the patient during tolvaptan treatment
The patient achieved remission with MMF and 3 cycles of methylprednisolone pulse therapy after this influenza infection and electrolyte abnormality improved. The serum creatinine was finally normalized, although it had become as high as 1.04 mg/dL during the course of treatment. TLV was temporarily suspended because the serum sodium level increased to 146 mEq/L on the 7th day after it was started. However, no other severe side effects were observed during treatment.
Discussion
This case showed the effectiveness of TLV for oliguria and edema, which were resistant to administration of an albumin preparation and furosemide, and it also improved hyponatremia. TLV is used extensively for adult patients with heart failure and hepatic edema; in particular, there is sufficient evidence of its effectiveness in cases with hyponatremia [1, 2, 4]. There are few reports of the use of TLV for NS in pediatric cases, although it has been tried as treatment for cardiac disease. Therefore, the appropriate dosage, treatment response, and safety of TLV are uncertain. Shimizu et al. [5] reported the effectiveness of TLV in a pediatric NS case that required extracorporeal ultrafiltration for massive edema. Meena et al. [6] also recently showed that combination therapy with TLV and furosemide was effective in pediatric NS patients with furosemide-resistant edema. In the present case, it was possible to avoid progression to a serious condition with early TLV treatment.
The effect of TLV against arginine vasopressin (AVP) is considered useful for the edema and oliguria of NS. Usually, the under-filling condition in NS stimulates AVP, which enhances reabsorption of free water in the medullary collecting ducts [7]. This reabsorption of free water is one of the factors that attenuates the effect of loop diuretics. Although the urinary sodium and FENa of this patient were slightly higher compared with those of the usual under-filling NS patient [8], it was determined that it resulted from inappropriate secretion of AVP caused by the combination of vomiting, infection, and volume depletion. After TLV, which has an anti-AVP effect, was administered, urinary volume increased rapidly, and edema improved.
In addition, TLV is also effective for hyponatremia in a patient with NS. NS patients are prone to hyponatremia due to further accumulation of free water, although the total amount of sodium increases with enhancement of sodium reabsorption. Increased urinary sodium excretion from the use of furosemide also exacerbates hyponatremia. Delbet et al. [9] reported the efficacy of TLV in a pediatric NS patient with severe hyponatremia. In the present case, TLV treatment decreased the concentration of U–Na + K and increased free water excretion. We considered that the improvement of hyponatremia in the present case was due to increasing excretion of free water.
The results of the EVEREST outcome trial involving adult patients with heart failure and serum sodium levels less than 134 mEq/L showed that the mean serum sodium concentration increased by 5.49 mEq/L, and these serum sodium levels reached a steady state in a week [1, 2]. Recently, it has been confirmed that TLV’s effect on urinary sodium excretion occurs through inhibition of vasopressin receptor 2, which represses epithelial Na-channel (ENaC) activity [4]. However, it has been reported that urinary sodium excretion in a patient with hyponatremia did not increase, and only the serum sodium level increased significantly during the use of TLV [10]. The present case had no other severe side effects, although there was a temporary increase of the serum sodium level. With careful monitoring of electrolytes, TLV appears safe and appropriate for fluid and electrolyte management in pediatric NS patients.
There are several reports that examined TLV efficacy. A responder to TLV usually has increased urine volume during the next 24 h on the first day, and decreased U-OSM can be used for monitoring [10, 11]. Furthermore, Imamura et al. [10] reported that more than a 26% decrease in U-OSM from a baseline > 352 mOsm/L for the first 4–6 h is an independent predictor of responders. U-OSM in the present case remained at lower levels than S-OSM during the use of TLV, although U-OSM was not measured before administering TLV; it is presumed that U-OSM decreased with TLV. In addition, TLV was useful for hypoalbuminemia in NS patients, just as it was reported to be effective in patients with hypoalbuminemia with hepatic edema [3].
With respect to the indications for administering TLV to pediatric NS patients, there is insufficient information. Through this case, it appears that refractory edema with hyponatremia is a good indication for TLV, after correction of intravascular volume with appropriate infusion solution and administration of an albumin preparation. Furthermore, frequent evaluation of fluid balance with urine and blood examinations and X-ray and ultrasonographic observations are needed to ensure safety during administration of TLV.
In conclusion, prompt TLV treatment is effective for the management of hyponatremia and fluid balance in NS patients with refractory edema resistant to other diuretics. Changes in urinary electrolytes and urinary osmolality can be used to predict the response to TLV and to assess the therapeutic effect of TLV; they can be used as biomarkers for monitoring, in addition to urine volume and decreased body weight. Further studies are needed to assess the effectiveness and safety of TLV in pediatric NS patients.
Declarations
Conflict of interest
No author has a direct conflict of interest that is relevant to this study. Outside the contents of the study, Yoshitsugu Kaku received travel fees from CLS Behring.
Human and animal rights
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nodari S, Jao GT, Chiong JR. Clinical utility of tolvaptan in the management of hyponatremia in heart failure patients. Int J Nephrol Renovasc Dis. 2010;3:51–60. doi: 10.2147/ijnrd.s7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 3.Sakaida I, Kawazoe S, Kajimura K, Saito T, Okuse C, Takaguchi K, Okada M, Okita K. Tolvaptan for improvement of hepatic edema: a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:73–82. doi: 10.1111/hepr.12098. [DOI] [PubMed] [Google Scholar]
- 4.Imamura T, Kinugawa K, Minatsuki S, Muraoka H, Kato N, Inaba T, Maki H, Hatano M, Yao A, Komuro I. Urine sodium excretion after tolvaptan administration is dependent upon baseline serum sodium levels: a possible explanation for the improvement of hyponatremia with scarce chance of hypernatremia by a vasopressin receptor antagonist. Int Heart J. 2014;55:131–137. doi: 10.1536/ihj.13-221. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu M, Ishikawa S, Yachi Y, Muraoka M, Tasaki Y, Iwasaki H, Kuroda M, Ohta K, Yachie A. Tolvaptan therapy for massive edema in a patient with nephrotic syndrome. Pediatr Nephrol. 2014;29:915–917. doi: 10.1007/s00467-013-2687-1. [DOI] [PubMed] [Google Scholar]
- 6.Meena J, Sinha A, Hari P, Bagga A. Therapy with the combination of tolvaptan and furosemide for refractory edema in nephrotic syndrome. Indian J Nephrol. 2020;30:53–55. doi: 10.4103/ijn.IJN_358_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Usberti M, Federico S, Meccariello S, Cianciaruso B, Balletta M, Pecoraro C, Sacca L, Ungaro B, Pisanti N, Andreucci VE. Role of plasma vasopressin in the impairment of water excretion in nephrotic syndrome. Kidney Int. 1984;25:422–429. doi: 10.1038/ki.1984.34. [DOI] [PubMed] [Google Scholar]
- 8.Kapur G, Valentini RP, Imam AA, Mattoo TK. Treatment of severe edema in children with nephrotic syndrome with diuretics alone-a prospective study. Clin J Am Soc Nephrol. 2009;4:907–913. doi: 10.2215/CJN.04390808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delbet JD, Parmentier C, Ulinski T. Tolvaptan therapy to treat severe hyponatremia in pediatric nephrotic syndrome. Pediatr Nephrol. 2020;35:1347–1350. doi: 10.1007/s00467-020-04530-6. [DOI] [PubMed] [Google Scholar]
- 10.Imamura T, Kinugawa K, Shiga T, Kato N, Muraoka H, Minatsuki S, Inaba T, Maki H, Hatano M, Yao A, Kyo S, Nagai R. Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patient -association between non-responders and chronic kidney disease. Circ J. 2013;77:397–404. doi: 10.1253/circj.CJ-12-0971. [DOI] [PubMed] [Google Scholar]
- 11.Shoaf SE, Bricmont P, Mallikaarjun S. Pharmacokinetics and pharmacodynamics of oral tolvaptan in patients with varying degrees of renal function. Kidney Int. 2014;85:953–961. doi: 10.1038/ki.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]