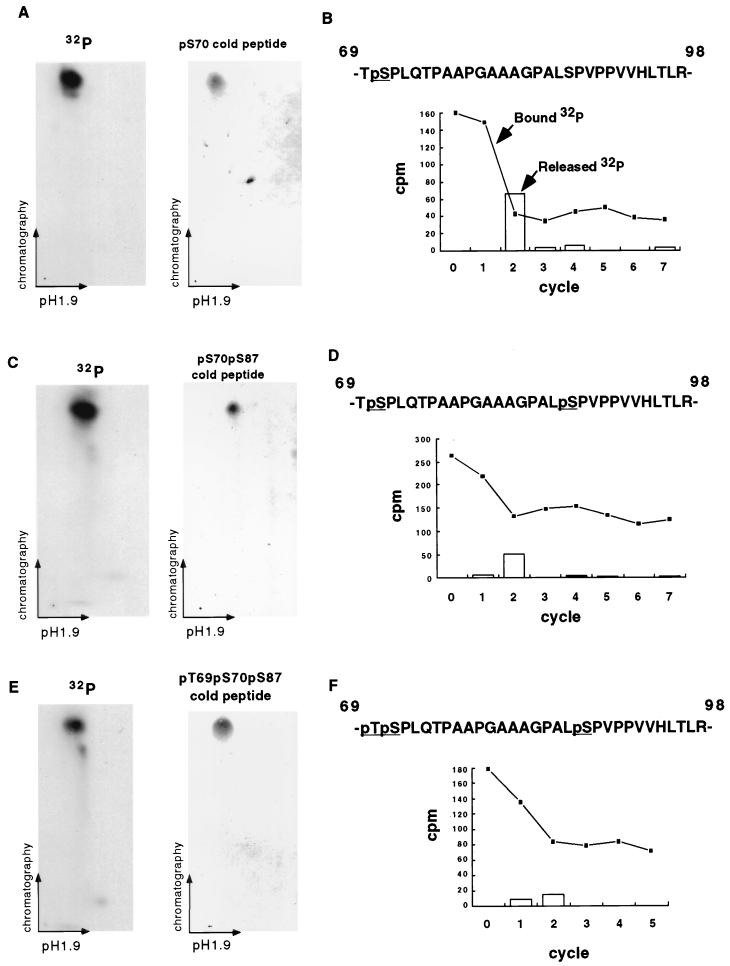
FIG. 2.
Paclitaxel induces phosphorylation of Ser70, Ser87, and Thr69 in BCL-2 (A) 2D mapping and synthetic phosphopeptide comigration study of band 1. BCL-2 was immunoprecipitated from 32P-labeled Jurkat-BCL-2 cells treated with paclitaxel, size fractionated by SDS-PAGE, and transferred to a nitrocellulose membrane. The tryptic peptides of band 1 (Fig. 1A) were separated on a TLC plate by electrophoresis at pH 1.9 and chromatography. A synthetic pS70 phosphopeptide corresponding to band 1 migrated and was visualized on a TLC plate by ninhydrin staining. Moreover, the admixture of the pS70 peptide with the tryptic peptides revealed comigration on TLC plates (not shown). (B) Manual sequencing of tryptic phosphopeptide from band 1. The 32P-labeled peptide was eluted from the TLC plate, conjugated to a Sequelon-AA membrane, and subjected to manual Edman degradation. The radioactivity on the membrane (closed squares) or released into the liquid (open bars) was measured at the end of each cycle. (C and D) 2D mapping, synthetic phosphopeptide migration, and manual sequencing of band 2 as described above. (E and F) 2D mapping of synthetic phosphopeptide migration and manual sequencing of band 3 as described above. Tryptic peptides derived from bands 1, 2, and 3 were also eluted from the radioactive spots on TLC plates (A, C, and E) and subjected to phosphoamino acid analysis, confirming their composition.