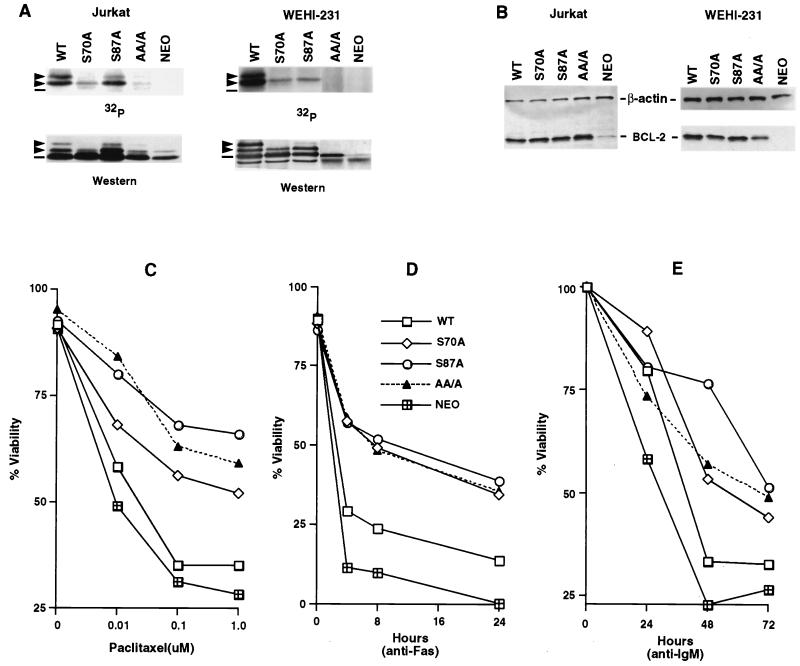
FIG. 3.
Substitution of phosphorylation sites in BCL-2 further enhances antiapoptotic activity. (A) Jurkat clones and WEHI-231 clones stably expressing WT or alanine-substituted phosphorylation sites of BCL-2, consisting of Ser70 (S70A), Ser87 (S87A), or all three phosphorylation sites, including Thr69 plus the two serines (AA/A), or an empty vector (Neo) were generated. Cells were treated with 1 μM paclitaxel for Jurkat clones or 1 μM vincristine for WEHI-231 clones and in vivo labeled with 32P-orthophosphate. BCL-2 was immunoprecipitated, separated by SDS-PAGE, and transferred to a membrane. After autoradiography, the same membranes were used for immunoblotting. Arrowheads denote phosphorylated BCL-2, and dashes denote nonphosphorylated BCL-2. The residual-shifted band on Western analysis of Jurkat cells bearing AA/A or Neo represents the endogenous hBCL-2 of Jurkat cells. (B) BCL-2 expression levels in Jurkat and WEHI-231 clones, determined by Western blot analysis of lysates from equivalent cells of various Jurkat or WEHI-231 clones with anti-hBCL-2 Ab 6C8 and anti-β-actin Ab. (C to E) Cell viability assays of Jurkat clones (C and D) or WEHI-231 clones (E). Jurkat clones expressing comparable WT, S70A, S87A, AA/A BCL-2, or Neo control vector were stimulated with various doses of paclitaxel for 24 h (C) or anti-Fas antibody (100 ng/ml) (D), while WEHI-231 clones were treated with anti-IgM Ab (BET-2 supernatant at 1:100 dilution) (E). Viability was determined by PI exclusion using flow cytometry. The results represent the means of triplicate assays. Another independent set of clones expressing matched levels of WT or mutant BCL-2 showed comparable results.