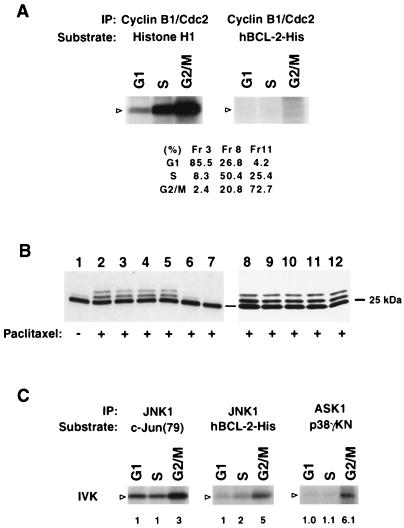
FIG. 5.
G2/M-activated ASK1-JNK1 pathway and JNK1 phosphorylation of BCL-2 in vitro. (A) G2/M-activated cyclin B1-Cdc2 complex does not phosphorylate BCL-2. Elutriated G1 (fraction [Fr] 3), S (Fr 8), and G2/M (Fr 11) fractions of Jurkat cells were lysed and immunoprecipitated (IP) with anti-cyclin B1. The phosphorylation of substrate histone H1 was assessed. The activity of this kinase complex for recombinant hBCL-2-His was also assessed. Arrowheads denote the position of the substrate. Cell cycle status was assayed by PI staining using a FACScan and CELLQUEST software. (B) Genistein and staurosporine inhibit paclitaxel-induced BCL-2 phosphorylation. Jurkat cells expressing WT BCL-2 were pretreated with various kinase inhibitors including 50 μM PD98059 (lane 3), 10 μM SB203580 (lane 4), 10 μM SB202190 (lane 5), 10 μg of genistein per ml (lane 6), 0.1 μM staurosporine (lane 7), 100 μM Rp-cAMP (lane 9), 10 μM LY294002 (lane 10), 1 μM wortmannin (lane 11), 20 ng of rapamycin per ml (lane 12), or DMSO (lanes 1, 2, and 8) for 60 min and then treated with (+) or without (−) paclitaxel for 6 h. BCL-2 phosphorylation was examined by Western blot analysis. (C) In vitro kinase (IVK) assay. The ASK1-JNK1 pathway is activated at the G2/M stage in cycling cells, and JNK1 phosphorylates BCL-2 in vitro. Lysates from elutriated fractions of Jurkat cells were immunoprecipitated with anti-ASK1 (DAV) serum or anti-JNK1 antibody. The ASK1 complex was incubated with GST-MKK6 and then with GST-p38γKN as a substrate to measure kinase activity. JNK activity was determined with GST–c-Jun (79) as a substrate. Recombinant hBCL-2-His was also incubated with the JNK1 complex. The position of substrates is denoted by open arrowheads. The fold activation of kinase activity is indicated as detected by phosphorimage analysis with activity at G1 set at 1.0. Western analysis of immunoprecipitates confirmed an equivalent amount of the kinase protein in each fraction (not shown).