Table 3. Kinetic Models and Adsorption Isotherms Used in the Present Studya.
| model | linear form | |
|---|---|---|
| kinetic models | pseudo-first-order | 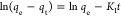 |
| pseudo-second-order | 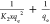 |
|
| Elovich | 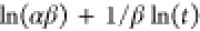 |
|
| goodness of adsorption (Chi-square) | 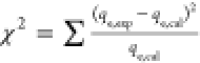 |
|
| adsorption isotherm | Langmuir isotherm | 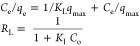 |
| Freundlich isotherm | 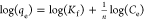 |
|
| Temkin | 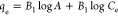 |
qe, qt, and qm are the amount of adsorbed dye at equilibrium, the amount of dye adsorbed at time t, and the maximum adsorption capacity (mg·g–1), respectively. K1 and K2 are the rate constant of pseudo-first-order (min–1) and rate constant of pseudo-second-order (g·mg–1·min–1), respectively. β is the desorption constant (g·mg–1) and α is the initial adsorption rate (mg·g–1·min–1). Ce is the equilibrium concentration (mg·L–1), KL is the adsorption equilibrium constant (L·mg–1), RL is the separation factor, B1 is the Temkin constant, and A is the equilibrium bond constant.
