Table 1. Optimization of the Catalyst Amount, Solvent, and Temperature for the Synthesis of 4aa.
| entry | catalyst (g) | solvent | temperature (°C) | time (min) | yield (%)b | conv (%)c |
|---|---|---|---|---|---|---|
| 1 | no catalyst | r.t. | 120 | trace | 0 | |
| 2 | 0.005 | EtOH | reflux | 60 | 45 | 70 |
| 3 | 0.01 | EtOH | reflux | 30 | 65 | 81 |
| 4 | 0.03 | EtOH | reflux | 30 | 77 | 95 |
| 5 | 0.05 | EtOH | reflux | 10 | 96 | 100 |
| 6 | 0.07 | EtOH | reflux | 20 | 80 | 100 |
| 7 | 0.1 | EtOH | reflux | 20 | 75 | 100 |
| 8 | 0.15 | EtOH | reflux | 30 | 71 | 99 |
| 9 | 0.05 | H2O | reflux | 30 | 55 | 100 |
| 10 | 0.05 | H2O:EtOH (1:1) | reflux | 45 | 73 | 100 |
| 11 | 0.05 | CH3OH | reflux | 60 | 70 | 97 |
| 12 | 0.05 | CH2Cl2 | reflux | 110 | 55 | 87 |
| 13 | 0.05 | CH3CN | reflux | 110 | 40 | 85 |
| 14 | 0.05 | toluene | reflux | 120 | 45 | 66 |
| 15 | 0.05 | DMF | reflux | 100 | 53 | 75 |
| 16 | no catalyst | EtOH | reflux | 120 | 10 | 15 |
| 17 | no catalyst | EtOH | r.t. | 120 | trace | 0 |
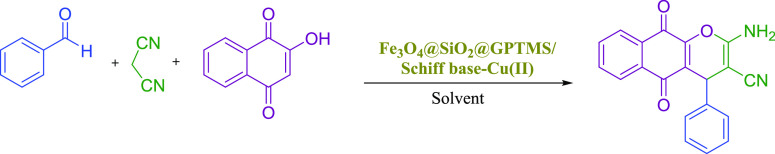
Reaction conditions: benzaldehyde (1 mmol), phenol (1 mmol), malononitrile (1 mmol), various solvents (2 mL).
Isolated yield.
Conversions were calculated from the 1H NMR spectrum of crude products.