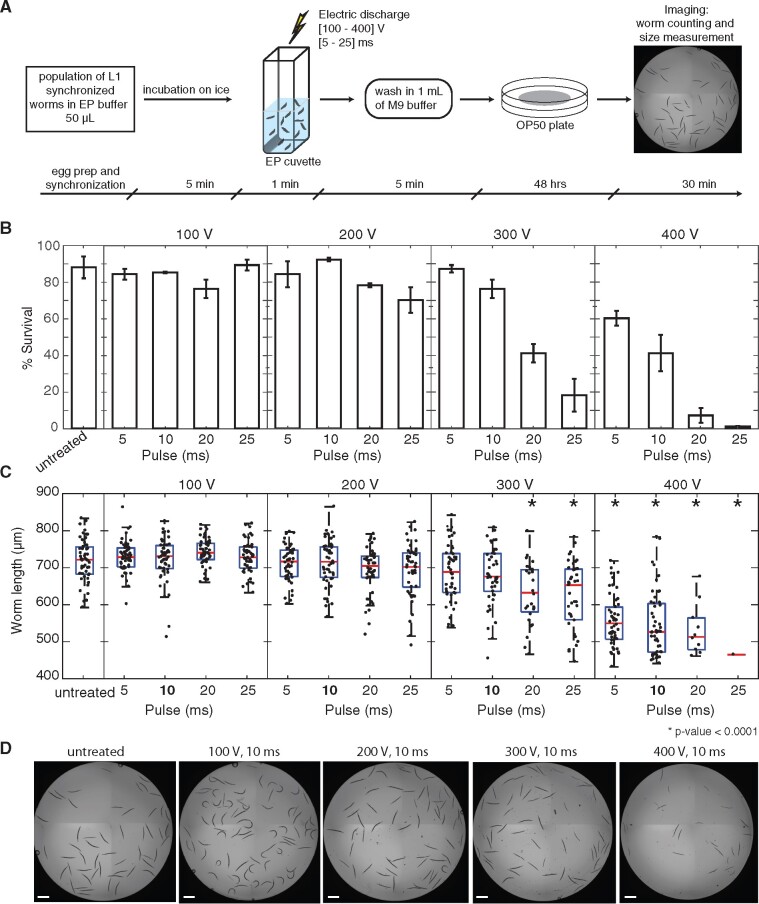
Figure 1.
Optimization of electroporation conditions for C. elegans viability. General pipeline (A) of the electroporation procedure starts with the preparation of L1 synchronized worms (∼250), which are then mixed with electroporation buffer 80% in chilled cuvettes. After electroporation, worms are washed with 1 mL of M9 buffer and collected by centrifugation at 500 rcf for 2 min, then transferred to E. coli OP50 seeded plates to grow at 20° for 48 h. Animal survival rates (B) and body lengths (C) varied based on the electroporation conditions applied. The evaluation was performed using N2 animals for each pair of electroporation parameters with an electroporation pulse duration ranging from 5 to 25 ms and voltage ranging from 100 to 400 V. For (b), data were normalized to the initial calculated input number of worms (number of L1 worms was microscopically counted in 5 μl of the stock suspension before distribution between the experimental tubes). Animals placed in electroporation buffer without electric discharge were used as “untreated” controls. Worm survival rates: mean ± SD (standard deviation)% of two independent experiments. Body length measurements: here and on all other figures, if not stated otherwise, red lines indicate means, blue boxes show 25th and 75th percentiles, whiskers show the data distribution range. *P-values < 0.05 were considered statistically significant (ANOVA test with Bonferroni correction). Representative images (D) of worm populations exposed to electric discharges of different voltages (10 ms pulses) demonstrate the pronounced effect of the electroporation procedure on animal viability. Scale bar = 500 μm.