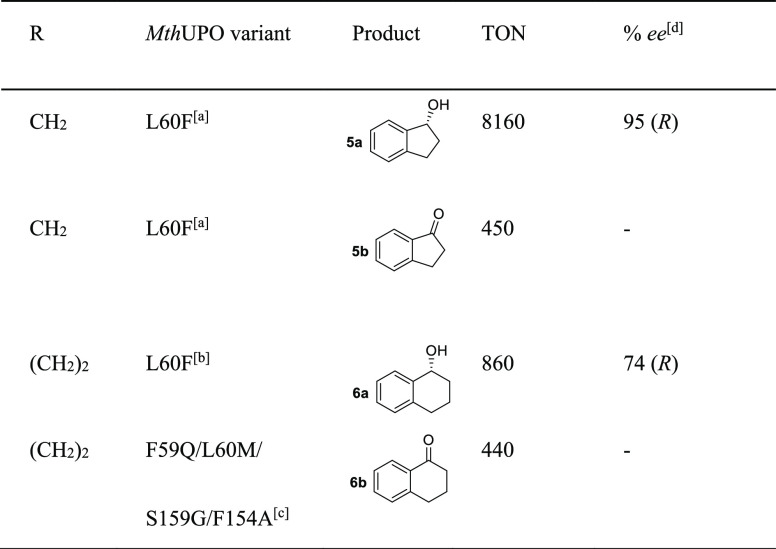
Table 3. Catalytic Activity of MthUPO Variants toward Benzylic Hydroxylation Yielding Chiral Productsa.
TON = turnover number, standard deviation <6.5%, reaction conditions: 100 nM MthUPO variant, 1 mM indane, 1 mM H2O2, 100 mM KPi buffer (pH 7), 5% acetone (v/v), 1 h at 25 °C in triplicates.
100 nM MthUPO variant, 1 mM 1,2,3,4-tetrahydronaphthalene, 1 mM H2O2, 100 mM KPi buffer (pH 7), 5% acetone (v/v), 1 h at 25 °C in triplicates.
100 nM MthUPO variant, 1 mM 1,2,3,4-tetrahydronaphthalene, 2 mM H2O2, 100 mM KPi buffer (pH 7), 5% acetone (v/v), 2 h at 25 °C in triplicates.
Determined by chiral GC.