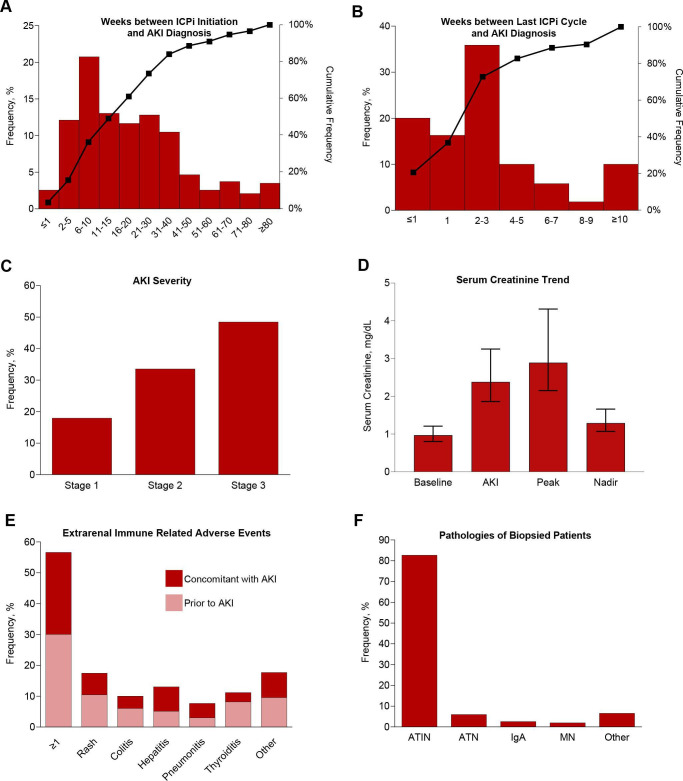
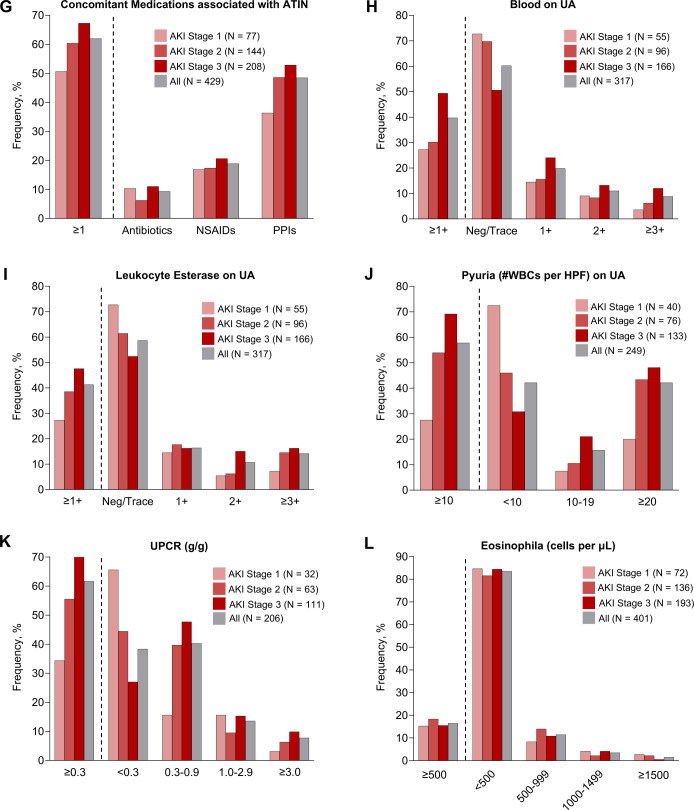
Figure 2.
Clinical features of ICPi-AKI. (A) The number of weeks between ICPi initiation and ICPi-AKI diagnosis. (B) The number of weeks between the last ICPi cycle and ICPi-AKI diagnosis. (C) Distribution of AKI severity. (D) Serum creatinine trend (median, IQR). (E) Frequency of extrarenal irAEs occurring before (>14 days) or concomitant (within 14 days before or after) with ICPi-AKI diagnosis. Other irAEs include hypophysitis (0.7% prior, 1.4% concomitantly), adrenalitis (0.2% prior, 1.4% concomitantly), type 1 diabetes mellitus (0% prior, 0.5% concomitantly), and myocarditis (1.2% prior, 0.2% concomitantly). (F) Distribution of pathologies among the 151 patients who underwent biopsy. Other includes 2 patients with FSGS and one patient with each of the following: reactive amyloidosis, AA amyloidosis, focal proliferative glomerulonephritis with C3 deposits, immune complex deposition disease not otherwise specified, mesangial proliferative immune complex mediated glomerulonephritis, pauci-immune glomerulonephritis, minimal change disease and thrombotic microangiopathy. (G) Frequency of potential ATIN-causing medications taken within 14 days before ICPi-AKI diagnosis. (H) Frequency of blood on UA at the time of ICPi-AKI. (I) Frequency of leukocyte esterase on UA at the time of ICPi-AKI. (J) Frequency of pyuria on UA at the time of ICPi-AKI. (K) Frequency of proteinuria at the time of ICPi-AKI. (L) Frequency of eosinophilia at the time of ICPi-AKI. AKI, acute kidney injury; ATIN, acute tubulointerstitial nephritis; ATN, acute tubular necrosis; FSGS, focal segmental glomerulosclerosis; HPF, high-power field; ICPi, immune checkpoint inhibitor; MN, membranous nephropathy; UA, urinalysis; UPCR, urine protein-to-creatinine ratio; WBCs, white blood cells.