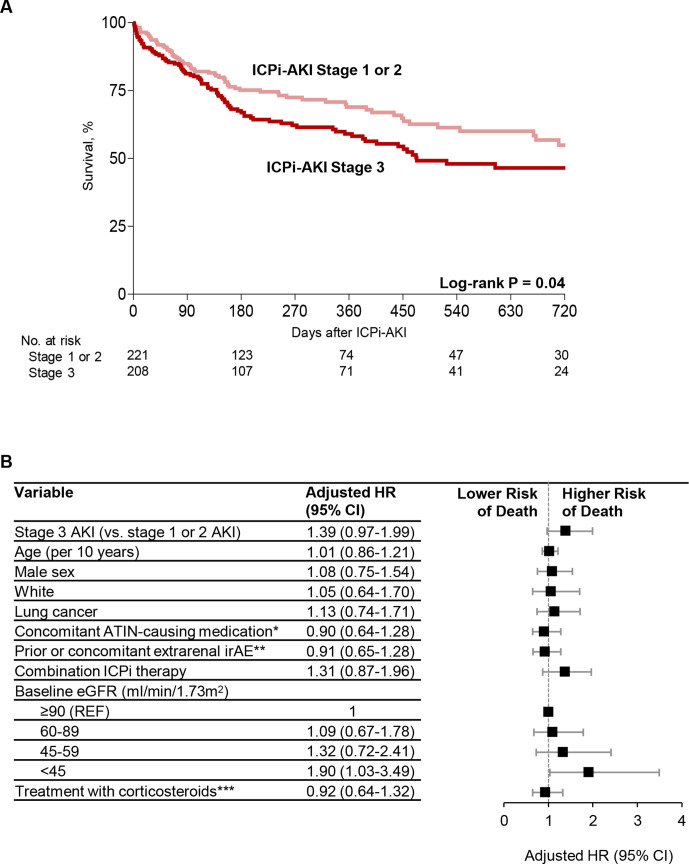
Figure 5.
Risk factors for death in patients with ICPi-AKI. (A) Survival among patients with stages 1 and 2 ICPi-AKI versus stage 3. (B) Multivariable Cox regression model showing predictors of death among patients with ICPi-AKI (total n=405, of whom 144 (35.6% (died)). Patients who died within 14 days of ICPi-AKI (n=24) were excluded. All model covariates are shown in the figure. *Denotes receipt of NSAIDs, PPIs, or antibiotics in the 14 days preceding ICPi-AKI. **Extrarenal irAEs were assessed prior to (>14 days) or concomitant (within 14 days before or after) with ICPi-AKI. ***Refers to oral or intravenous corticosteroids initiated within 14 days following ICPi-AKI. AKI, acute kidney injury; ATIN, acute tubulointerstitial nephritis; eGFR, estimated glomerular filtration rate; ICPi, immune checkpoint inhibitor; irAEs, immune-related adverse events; NSAIDs, non-steroidal anti-inflammatory drugs; PPI, proton pump inhibitor.