Figure 5.
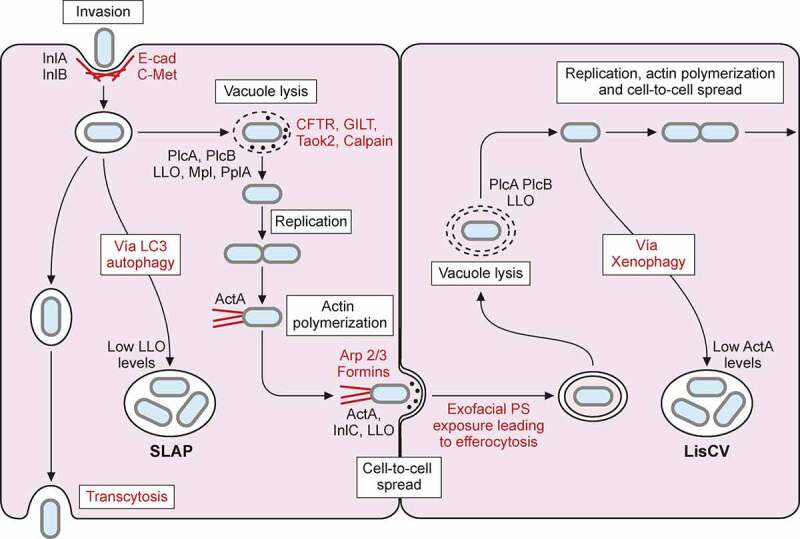
L. monocytogenes intracellular lifecycle. Invasion of non-phagocytic cells mediated by InlA and InlB interaction with host receptors E-cadherin (E-cad) and C-Met respectively, enhances actin polymerization and leads to bacterial internalization. Once inside a primary vacuole in host cytoplasm, L. monocytogenes can follow different pathways. Bacterium remains in vacuole, leading to transcytosis, as in goblet cells. In some macrophages, L. monocytogenes can replicate inside this vacuole, developing spacious Listeria containing phagosomes (SLAPs), whose formation is associated with autophagy and low LLO secretion. The vacuole can be lysed by virulence factors LLO, PlcA, PlcB, Mpl and PplA. Release of LLO into the cytoplasm has different effects on host cell, like histone modifications, mitochondrial fission, etc. In the cytoplasm of trophoblasts and hepatocytes, L. monocytogenes can be engulfed into an acidic vacuole known as Listeria containing vacuole (LisCV). Formation of LisCV’s may be due to xenophagy process in host cell and loss of ActA in L. monocytogenes. Listeria in the cytoplasm induces ActA, which interacts with host Arp 2/3 complex and formins. This promotes actin polymerization, which propels Listeria throughout the cytoplasm and leads to protrusion formation on adjacent cell. Internalin C (InlC) secretion in host cell cytoplasm perturbs apical junctions, facilitating cell-to-cell spread. LLO, also secreted in the protrusion, damages host cell membrane, exposing inner phosphatidylserine in exoplasmic layer of protrusion membrane. Exofacial exposure of phosphatidylserine is recognized as an eat-me signal that promotes Listeria-containing vesicle engulfment by macrophages. Therefore, L. monocytogenes also exploits efferocytosis for cell-to-cell spread. Bacterium will be hosted in new cell within double membrane vacuole that can be lysed again, repeating its infectious lifecycle
