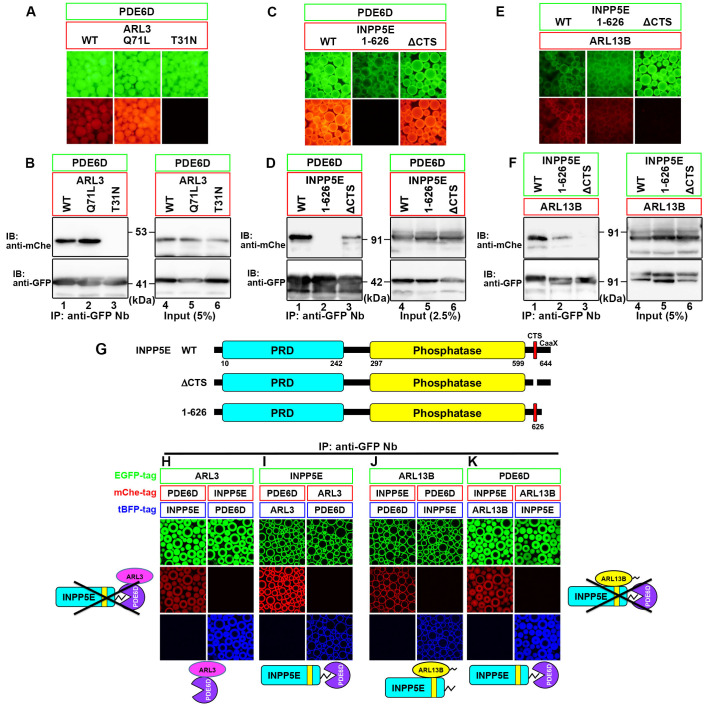
Fig. 1.
Mutually exclusive binding of ARL3 and INPP5E to PDE6D, and that of PDE6D and ARL13B to INPP5E. (A–F) HEK293T cells were cotransfected with expression vectors for EGFP-PDE6D and an mChe-fused ARL3 construct as indicated (A, B), for EGFP-PDE6D and an mChe-fused INPP5E construct as indicated (C, D), or for ARL13B-mChe and EGFP-fused INPP5E construct as indicated (E, F). Lysates prepared from the transfected cells were subjected to immunoprecipitation with GST-fused anti-GFP Nb prebound to glutathione-Sepharose beads, and the precipitated beads bearing fluorescent fusion proteins were observed under a microscope (A, C, E). Proteins bound to the beads were then subjected to SDS-PAGE, followed by immunoblotting analysis using anti-mChe and anti-GFP antibodies (B, D, F). (G) Schematic representation of the domain organization of INPP5E and its mutants used in this study. PRD, proline-rich domain. (H–K) Lysates were prepared from cells cotransfected with expression vectors for the indicated combinations of EGFP-fused, mChe-fused, and tBFP-fused constructs; to express approximately equal amount of the ARL3, INPP5E, PDE6D, and ARL13B proteins fused with EGFP, mChe, or tBFP, HEK293T cells were transfected with the expression vectors for ARL3, INPP5E, and PDE6D at the ratio of 1:6:1 (H, I), or those for ARL13B, INPP5E and PDE6D at the ratio of 4:5:1 (J, K). The lysates were then immunoprecipitated with GST–anti-GFP Nb, followed by observation under a microscope. Expected interactions are shown schematically.