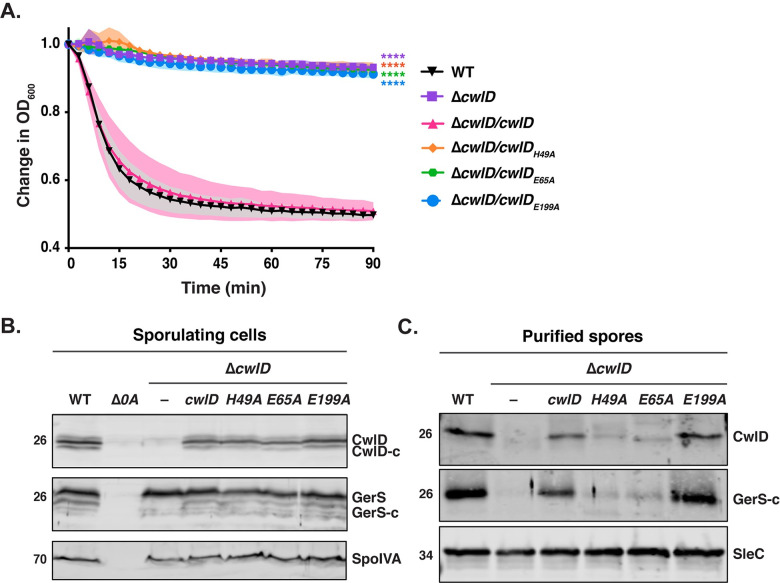
Fig 6. Mutations in Zn2+ binding residues disrupt CwlD function and affect the stability of the CwlD:GerS complex in the spore.
(A) Spore germination as measured by the change in the OD600 in response to germinant in cwlD mutants spores relative to wild-type spores. Purified spores were resuspended in BHIS, and germination was induced by adding taurocholate (1% final concentration). The ratio of the OD600 of each strain at a given time point relative to the OD600 at time zero is plotted. The mean OD600 determined from three biological replicates from three independent spore preparations are shown. Shading represents the standard deviation as the area between error bars for each time point measured. Statistical significance relative to wild-type was determined using two-way ANOVA and Tukey’s test. **** p < 0.0001. (B) Western blot analysis of CwlD and GerS levels in sporulating cells from wild type (WT), ΔcwlD, and ΔcwlD strains complemented with constructs encoding the CwlD variants H49A, E65A and E199A. His49 and Glu65 directly bind Zn2+ in the structure, while Glu199 is part of the amidase catalytic mechanism. CwlD-c and GerS-c indicate the cleaved form of both proteins. Δspo0A (Δ0A) was used as negative control for sporulating cells, and SpoIVA was used as a loading control. (C) Western blot analysis of CwlD and GerS levels in spores from the strains used in the experiments described in (A). SleC was used as a loading control.