Abstract
Aims
Oxidative stress plays an important role in the development and progression of heart failure (HF). Although exercise and oxidative stress are closely related, the effect of acute exercise on reactive oxygen species production and the fluctuation on prognosis are unclear.
Methods and results
We enrolled 94 patients who were hospitalized for worsening HF (mean age 68.0 ± 14.5 years old, 63.8% male). The changes in diacron‐reactive oxygen metabolites (d‐ROM) values, a marker of oxidative stress, before and after a cardiopulmonary exercise test were considered as Δd‐ROM. The mean follow‐up period was 24 ± 13 months, during which 15 patients had all‐cause death or left ventricular assist system implantation. Kaplan–Meier analysis demonstrated that all‐cause death or left ventricular assist system implantation was significantly higher in the Δd‐ROM‐positive group than in the Δd‐ROM‐negative group (log‐rank P = 0.047). Elevated Δd‐ROM levels were associated with increased mortality risk. Multivariate analysis adjusted for body mass index and peak oxygen uptake revealed that Δd‐ROM was an independent prognostic factor of adverse events (Tertile 3 vs. 1; hazard ratio: 4.57; 95% confidence interval: 1.21–29.77; P = 0.022).
Conclusions
Patients with HF who underwent a cardiopulmonary exercise test and had an increased oxidative stress marker level had a poor prognosis. The appropriate exercise intensity could be determined by evaluating the changes in oxidative stress status in response to acute exercise in patients with HF.
Keywords: Heart failure; Oxidative stress; Exercise intensity; Prognosis
Introduction
Heart failure (HF) has reached epidemic proportions worldwide, and it is one of the most common causes of hospitalization. 1 Although available medications and non‐pharmacological therapies have been developed, the number of patients with HF has increased. 2 Furthermore, HF has a high mortality rate and a very high rehospitalization rate, especially in elderly patients, which is becoming a major social problem. 3
Exercise tolerance has been demonstrated to be a more important prognostic indicator than cardiac function, such as left ventricular ejection fraction (EF), in patients with HF. 4 Exercise therapy is recognized as an effective intervention to prevent the onset and progress of HF and is recommended by various clinical guidelines. 5 , 6 The beneficial effects of exercise therapy were thought to be mediated by peripheral and central mechanisms, such as improvement of skeletal muscle quality and function, endothelial function in peripheral vessels and neurohumoral interaction. However, these changes could not explain all the mechanisms of the beneficial effects of exercise; thus, other mechanisms are suggested to be involved. 7 Considering the mode of exercise, mild‐to‐moderate intensity aerobic exercise was performed as exercise therapy for patients with HF for a long time; however, the effect of aerobic interval training has also been demonstrated in recent years. 8 There is still controversy regarding the level and format of exercise that can yield optimal beneficial effects.
Oxidative stress is defined as an imbalance between reactive oxygen species (ROS) production and antioxidant defences. The impairment of cellular function induced by oxidative stress results in the onset and progression of various diseases. In the heart, oxidative stress induces myocyte hypertrophy, cell apoptosis and calcium overload by oxidized membrane phospholipids, proteins and DNA. 9 , 10 It has been clinically shown that patients with HF are exposed to oxidative stress even at rest 11 , 12 and oxidative stress plays important roles in the development and progression of HF. 9 In its physiological aspect, transient and moderate production of ROS acts as a signal transduction factor that regulates energy metabolism and protein synthesis and contributes to the adaptation of physiological functions caused by moderate exercise. 13 These physiological functions of ROS are called ‘hormesis effects’. 13 An important relationship is suggested between exercise and oxidative stress; however, the effect of acute, high‐intensity aerobic exercise on ROS production and fluctuation has not been evaluated yet. In addition, their impact on prognosis in patients with HF is unclear. 9 , 14 This study investigated whether changes in the value of derivatives of reactive oxidative metabolites (e.g. diacron‐reactive oxygen metabolites [d‐ROM]), an oxidative stress marker, during a symptom‐limited exercise stress test (e.g. cardiopulmonary exercise test [CPX]) could predict prognosis in patients with HF.
Methods
Study population
We prospectively enrolled patients who were hospitalized with worsening HF and diagnosed with one or two major criteria in conjunction with two minor Framingham criteria by at least two independent cardiologists at the Osaka City University Graduate School of Medicine between July 2013 and March 2015. 15 Exclusion criteria for this study were as follows: (1) patient experienced acute coronary syndrome within the preceding 30 days; (2) experienced open heart surgery within the preceding 3 months; (3) underwent percutaneous coronary angioplasty or cardiac resynchronization therapy during hospitalization; (4) the presence of severe valvular heart disease; (5) being on dialysis; (6) cannot enforce CPX due to musculoskeletal problems or paralysis; and (7) unwillingness to provide informed consent. For patients who did not satisfy the exclusion criteria, cardiac rehabilitation was introduced after standard treatment. A total of 94 patients with New York Heart Association (NYHA) Classes I–III were registered.
Demographic, laboratory and echocardiographic data were collected from the patients' medical records at the time of enrolment in this study. The study protocol was approved by the institutional ethics committee of Osaka City University (approval number: 2569) and was conducted in accordance with the recommendations of the 1975 Declaration of Helsinki. Written informed consent was obtained from all patients.
Study design and cardiac rehabilitation
This was a single‐centre, prospective cohort study. After informed consent was obtained from all patients, they underwent cardiac rehabilitation during hospitalization. Most patients underwent CPX during the first period of cardiac rehabilitation to determine their anaerobic threshold (AT) using the V‐slope method. 16 The patients' cardiac rehabilitation strength was set based on the AT value determined by the initial CPX. For patients who had difficulty undergoing CPX at the start of rehabilitation, their rehabilitation strength was set using the Karvonen equation. Karvonen's coefficient was determined at 0.4–0.6. 5 Patients underwent supervised exercise combining aerobic and resistance training in a 30 min/session, which was performed five times per week. CPX was performed to evaluate exercise tolerance prior to discharge, and guidance was given on exercise therapy after discharge.
The patients were prospectively followed up until March 2017. The primary endpoints of the study were the incidence of all‐cause death or left ventricular assist system (LVAS) implantation, whereas the secondary endpoints were the incidence of HF death or rehospitalization with worsening HF. If both events occurred, only the first event was taken into consideration. In Japan, LVAS implantation is only allowed in patients waiting for heart transplantation and who have been judged to have no other means of saving their lives other than heart transplantation, which is determined after examination by a third‐party organization; therefore, death and LVAS implantation are sometimes treated as equivalent. 17
CPX
Most patients underwent CPX during the first period of cardiac rehabilitation, and all patients underwent CPX immediately before discharge. The exercise stress tests were performed on an upright cycle ergometer (Strength Ergo 8; Fukuda Denshi, Tokyo, Japan) using a ramp protocol. The ramp protocol consisted of 4‐min rest on the cycle ergometer, starting at 0 or 10 W for a 4‐min warm‐up and followed by an incrementally increasing work rate of 10 W/min to maximum tolerance. Although several criteria exist for assessing maximal exercise effort, the peak respiratory exchange ratio (RER) was used as an objective criterion of effort. Peak RER ≥ 1.10 is accepted to be indicative of maximal effort, based on current guidelines. 18 In this study, we set the load stop condition at peak RER ≥ 1.10. The electrocardiogram was monitored continuously, and blood pressure was recorded every minute before, during and after exercise (ML‐9000; Fukuda Denshi). Expired gas analysis was performed with a breath‐by‐breath method using an expired gas analyser (Cpex‐1; Inter Reha, Tokyo, Japan). Oxygen uptake (VO2), carbon dioxide production (VCO2) and minute ventilation (VE) were measured before, during and after exercise. AT was determined using the V‐slope method. 16 Peak oxygen uptake (peak VO2) was defined as the highest VO2 value achieved at peak exercise. The slope of the relationship between VE and VCO2 (VE/VCO2 slope) was obtained by linear regression analysis during exercise prior to respiratory compensation.
d‐ROM measurement
An accurate measurement of in vivo oxidative stress is particularly challenging because of its potential unreliability and inaccuracy. Therefore, ROS are often evaluated by measuring products in blood or urine as a surrogate maker. 19 The d‐ROM test measures metabolites produced by oxidative stress in vivo and mainly reflects hydroperoxide (ROOH) levels in the blood. The d‐ROM level is high in patients with HF, with higher levels in severe cases as evaluated by NYHA functional class. 20
Blood samples for analysis of d‐ROM were collected just before and immediately after CPX immediately before discharge. After centrifugation at 4°C and 3000 rpm for 15 min, serum samples were stored at −80°C until analysis. Measurements of d‐ROM in serum were performed using a free radical analyser (FREE Carpe Diem; Wismerll, Tokyo, Japan). The measurement principle is based on the radicals (RO, alkoxyl radical; ROO, peroxy radical) produced from the decomposition of ROOH in blood by the Fenton reaction in the presence of iron, which oxidizes alkyl‐substituted aromatic amines to form coloured derivatives. The ROOH levels in blood were evaluated by measuring this absorbance with a dedicated measuring instrument. The measurements were performed according to the d‐ROM kit protocol (DI‐003b; Wismerll). The results were expressed in conventional Carratelli units (U.CARR). 21 One U.CARR unit corresponds to 0.8 mg/L of hydrogen peroxide. The normal reference level of d‐ROM is 250–300 U.CARR. 22 , 23 d‐ROM values above 300 U.CARR indicate a condition of oxidative stress. Δd‐ROM indicates an alteration in the equilibrium between pro‐oxidant and antioxidant capability during a symptom‐limited exercise stress test, which was calculated by subtracting the d‐ROM level before CPX from that after CPX.
Statistics
Continuous variables were shown as mean ± standard deviation or median with interquartile range for non‐normally distributed variables. The normality of the data was evaluated using the Shapiro–Wilk normality test. The patients were divided into Δd‐ROM‐positive or Δd‐ROM‐negative groups. To compare each parameter between the Δd‐ROM‐positive and Δd‐ROM‐negative groups, we used an unpaired t‐test for the normally distributed data, Wilcoxon–Mann–Whitney test for the not normally distributed data or Fisher's exact test for categorical variables. The Spearman correlation coefficient between Δd‐ROM and each continuous variable was calculated. The Kaplan–Meier curves were constructed for the time to death or LVAS implantation, and the log‐rank test was used for initial comparison. Univariate and multivariate Cox proportional hazard analyses were used to analyse predictors of cardiac events with adjusted confounding factors. The univariate Cox proportional hazard analysis was performed using 24 clinical variables such as generally recognized parameters influencing HF, CPX parameters, the d‐ROM level before CPX and Δd‐ROM. Furthermore, among the factors suggested to be predictors of cardiac events in univariate analysis, factors including peak VO2, which is an index of exercise tolerance, were input for multivariate analysis. The results of Cox proportional hazard models were presented as hazard ratios (HRs) and 95% confidence intervals (CIs). The reference change of Δd‐ROM for HR is per 1 U.CARR increase. Because the measurement results of d‐ROM levels were normally distributed, it is not necessary to transform into logarithmic or square root conversion. All analyses were performed used JMP software (v. 13; SAS Institute, Cary, NC, USA). A P‐value < 0.05 was considered statistically significant.
Results
Baseline characteristics
The baseline demographics and clinical characteristics of the study participants are shown in Table 1 . The mean age of the population was 68 years, and 64% of the patients were men. Eleven (11.7%) were current smokers, but these patients did not smoke during their hospitalization. The causes of HF included non‐ischaemic heart disease (n = 78, 83%) and ischaemic cardiomyopathy (n = 16, 17%). On admission, only 43.6% of patients were receiving β‐blockers, but 79.8% of patients were prescribed them at discharge. The d‐ROM levels of the sample before CPX had an intra‐assay coefficient of variation of 0.52 and an inter‐assay coefficient of variation of 0.25. The detection range of this measurement system was 40–1000 U.CARR, and nothing outside this detection range was found in present study. The mean serum d‐ROM level before CPX was 453.0 ± 111.0 U.CARR. These d‐ROM levels were much higher than the normal range, suggesting that patients with HF were exposed to high oxidative stresses even at rest. The median change in serum d‐ROM levels after CPX (Δd‐ROM) was −9.5 U.CARR, with 52 subjects having decreasing levels (d‐ROM‐negative group) and 42 subjects had increasing levels (Δd‐ROM‐positive group). The mean follow‐up period was 24 ± 13 months, during which 15 patients had all‐cause death or LVAS implantation.
Table 1.
Comparison of baseline characteristics of study patients based on serum Δd‐ROM levels
| Total | Negative Δd‐ROM group | Positive Δd‐ROM group | P‐value | |
|---|---|---|---|---|
| (n = 94) | (n = 52) | (n = 42) | ||
| Male (%) | 60 (63.8) | 32 (61.5) | 28 (66.7) | 0.669 |
| Age | 68.0 ± 14.5 | 67.5 ± 14.6 | 68.7 ± 14.6 | 0.685 |
| NYHA Class III (%) | 8 (8.5) | 4 (7.7) | 4 (9.5) | 0.752 |
| NYHA Class I/II (%) | 86 (91.5) | 48 (92.3) | 38 (90.5) | 0.752 |
| Current smoker (%) | 11 (11.7) | 7 (13.5) | 4 (9.5) | 0.749 |
| Comorbidity | ||||
| Hypertension (%) | 42 (44.7) | 23 (44.2) | 19 (45.2) | 0.922 |
| Diabetes mellitus (%) | 28 (29.8) | 19 (36.5) | 9 (21.4) | 0.121 |
| Atrial fibrillation (%) | 36 (38.3) | 17 (32.7) | 19 (45.2) | 0.286 |
| General examination | ||||
| BMI, kg/m2 | 22.2 ± 4.0 | 22.8 ± 4.4 | 21.4 ± 3.5 | 0.120 |
| Heart rate, bpm | 75.1 ± 13.6 | 74.8 ± 11.7 | 75.4 ± 15.8 | 0.858 |
| Resting systolic BP, mmHg | 108.0 ± 21.5 | 110.0 ± 17.5 | 107.6 ± 21.0 | 0.540 |
| Resting diastolic BP, mmHg | 60.9 ± 12.4 | 61.9 ± 11.4 | 59.5 ± 13.6 | 0.360 |
| Aetiology | ||||
| Ischaemic (%) | 16 (17.0) | 10 (19.2) | 6 (14.3) | 0.590 |
| LVEF ≥ 50% (%) | 28 (29.8) | 12 (23.1) | 16 (38.1) | 0.173 |
| Medication at discharge | ||||
| ACE inhibitor or ARB (%) | 59 (62.8) | 34 (65.4) | 25 (59.5) | 0.669 |
| β‐Blocker (%) | 75 (79.8) | 44 (84.6) | 31 (73.8) | 0.209 |
| Aldosterone receptor antagonist (%) | 44 (46.8) | 20 (38.5) | 24 (57.1) | 0.097 |
| Loop diuretic (%) | 69 (73.4) | 39 (75.0) | 30 (71.4) | 0.815 |
| Baseline use of devices | ||||
| Cardioverter‐defibrillator (%) | 4 (4.3) | 3 (5.8) | 1 (2.4) | 0.418 |
| Biventricular pacemaker (%) | 3 (3.2) | 2 (3.9) | 1 (2.4) | 0.688 |
| Echocardiogram | ||||
| LVEDD, mm | 55.2 ± 9.8 | 55.2 ± 8.6 | 55.2 ± 11.2 | 0.984 |
| LVESD, mm | 43.0 ± 13.1 | 43.8 ± 11.2 | 42.0 ± 15.2 | 0.547 |
| LVEF, % | 38.4 ± 14.8 | 37.5 ± 13.6 | 39.5 ± 16.3 | 0.535 |
| LAV, mL | 63.8(51.2–86.5) | 60.7(49.0–88.1) | 70.2(55.9–86.2) | 0.163 |
| E/e′ ratio | 20.5 ± 10.1 | 20.7 ± 9.3 | 20.2 ± 11.2 | 0.816 |
| Mitral regurgitation | 1.0 ± 0.8 | 0.9 ± 0.8 | 1.0 ± 0.9 | 0.745 |
| TRPG, mmHg | 28.0 (22.0–36.0) | 27.1 (21.3–34.0) | 30.4 (23.5–37.0) | 0.138 |
| Laboratory data | ||||
| Haemoglobin, g/dL | 12.5 ± 2.3 | 12.2 ± 2.2 | 12.8 ± 2.4 | 0.273 |
| Serum sodium, mEq/L | 139.5 ± 2.9 | 140.0 ± 3.0 | 138.8 ± 2.8 | 0.042 |
| eGFR, mL/min/1.73m2 | 50.6 ± 20.8 | 51.8 ± 22.4 | 49.0 ± 18.9 | 0.524 |
| Log BNP on admission | 2.6 ± 0.5 | 2.6 ± 0.5 | 2.7 ± 0.4 | 0.595 |
| Troponin T, ng/mL | 0.017 (0.010–0.042) | 0.019 (0.010–0.050) | 0.017 (0.011–0.034) | 0.665 |
| d‐ROM at pre CPX, U.CARR | 453.0 ± 111.0 | 466.2 ± 120.2 | 436.8 ± 97.5 | 0.203 |
| d‐ROM at post CPX, U.CARR | 448.8 ± 115.9 | 418.1 ± 116.6 | 486.8 ± 104.3 | 0.004 |
| Δd‐ROM, U.CARR | −9.5 (−41.3–33.5) | −31.0 (−55.3 ‐ ‐10.3) | 40.5 (18.8–60.3) | < 0.001 |
Values are mean ± standard deviation, median (interquartile range) or n (%).
ACE, angiotensin converting enzyme; ARB, angiotensin type 1 receptor blocker; BMI, body mass index; BNP, B‐type natriuretic peptide; BP, blood pressure; d‐ROM, derivatives of reactive oxidative metabolites; eGFR, estimated glomerular filtration rate; LAV, left atrial volume; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; NYHA, New York Heart Association; TRPG, tricuspid regurgitation pressure gradient.
We divided the patients into Δd‐ROM‐positive or Δd‐ROM‐negative groups. The baseline characteristics were compared between the Δd‐ROM‐positive and Δd‐ROM‐negative groups (Table 1 ). Considering the groups' demographics, the body mass index (BMI) tended to be lower in the Δd‐ROM‐positive group, but it did not reach statistical significance. The laboratory and echocardiogram data were not different between the two groups except for serum sodium levels. The serum d‐ROM levels before CPX did not differ between the two groups. Table 2 shows the exercise parameters in the CPX. In this study, we set the load stop condition at peak RER ≥ 1.10. In actuality, peak RER ≥ 1.10 could not be achieved in 16 cases (17.0%) because of strong subjective symptoms or weakness of lower limb muscles in patients. However, all the cases that could not achieve peak RER ≥ 1.10 had peak RER of 1.05–1.09, and it was judged that the maximal effort was acceptable. 24 The AT, peak VO2 and VE/VCO2 slope did not differ between the two groups.
Table 2.
Cardiopulmonary exercise test data at discharge
| Negative Δd‐ROM Group | Positive Δd‐ROM Group | P‐value | |
|---|---|---|---|
| (n = 52) | (n = 42) | ||
| Peak work rate, W | 55.0 (40.0–71.0) | 53 (40.0–70.0) | 0.888 |
| Peak respiratory exchange ratio | |||
| ≥1.10 (%) | 43 (82.7) | 35 (83.3) | 0.935 |
| 1.05–1.09 (%) | 9 (17.3) | 7 (16.7) | 0.935 |
| Anaerobic threshold, mL/kg/min | 13.0 ± 2.8 | 13.3 ± 2.5 | 0.664 |
| Anaerobic threshold (%predicted) | 88.9 ± 18.9 | 91.0 ± 16.2 | 0.576 |
| Peak oxygen uptake, mL/kg/min | 17.3 ± 4.4 | 17.7 ± 3.6 | 0.653 |
| Peak oxygen uptake (%predicted) | 74.9 ± 17.4 | 77.4 ± 15.4 | 0.475 |
| VE/VCO2 slope | 32.3 (27.3–35.5) | 32.6 (29.4–37.5) | 0.645 |
| ⊿HR/⊿WR | 0.46 (0.31–0.63) | 0.51 (0.37–0.65) | 0.732 |
Values are mean ± standard deviation.
HR, heart rate; VCO2, carbon dioxide production; VE, minute ventilation; WR, work rate.
Correlations between d‐ROM levels and clinical parameters
We evaluated the correlations between serum d‐ROM levels before CPX and each continuous variable (Table S1 ). A single regression analysis showed a slightly negative correlation between serum d‐ROM levels before CPX and AT (r = −0.230; P = 0.027), but there was no significant correlation with other clinical parameters. We also evaluated the correlation between Δd‐ROM levels and various clinical indicators (Table 3 ). Spearman's correlation coefficient analysis showed that Δd‐ROM levels were not significantly correlated with any demographic, laboratory or echocardiography findings. Furthermore, no significant correlation was observed between Δd‐ROM levels and exercise parameters.
Table 3.
Spearman correlation coefficient analysis between serum Δd‐ROM levels and each continuous variable
| Spearman r | P‐value | |
|---|---|---|
| Age | 0.171 | 0.100 |
| BMI, kg/m2 | −0.131 | 0.218 |
| Heart rate, bpm | 0.054 | 0.606 |
| Resting systolic BP, mmHg | −0.075 | 0.474 |
| Resting diastolic BP, mmHg | −0.142 | 0.173 |
| LVEDD, mm | −0.066 | 0.529 |
| LVESD, mm | −0.114 | 0.274 |
| LAV, mL | 0.083 | 0.434 |
| LVEF, % | 0.068 | 0.514 |
| E/e′ | −0.047 | 0.686 |
| Anaerobic threshold, mL/kg/min | 0.093 | 0.377 |
| Peak oxygen uptake, mL/kg/min | 0.094 | 0.368 |
| VE/VCO2 slope | 0.116 | 0.273 |
| Haemoglobin, g/dL | 0.088 | 0.397 |
| Serum sodium, mEq/L | −0.115 | 0.272 |
| eGFR, mL/min/1.73m2 | 0.083 | 0.427 |
| Log BNP on admission | 0.048 | 0.650 |
| Troponin T, ng/L | −0.007 | 0.949 |
Association between Δd‐ROM levels and cardiac events
Kaplan–Meier analysis showed that patients from the Δd‐ROM‐positive group had a higher risk of death or LVAS implantation compared with patients from the Δd‐ROM‐negative group (log‐rank test, P = 0.047) (Figure 1 A ). A similar trend was also indicated for HF death or readmission due to worsening HF (log‐rank test, P = 0.006) (Figure 1 B ). The Δd‐ROM levels were divided into tertiles, and additional analyses were conducted. As a result, Kaplan–Meier analysis revealed many cardiac events in the highest Δd‐ROM tertile compared with the lower two tertiles of Δd‐ROM levels (Figure 2 ). An analysis with only all‐cause death as the endpoint has also been performed; the highest Δd‐ROM tertile had more events than the lower two tertiles of Δd‐ROM levels (Figure S1 ). These results suggest that the prognosis worsens when the balance between pro‐oxidant and antioxidant capability is largely inclined to pro‐oxidants during CPX.
Figure 1.
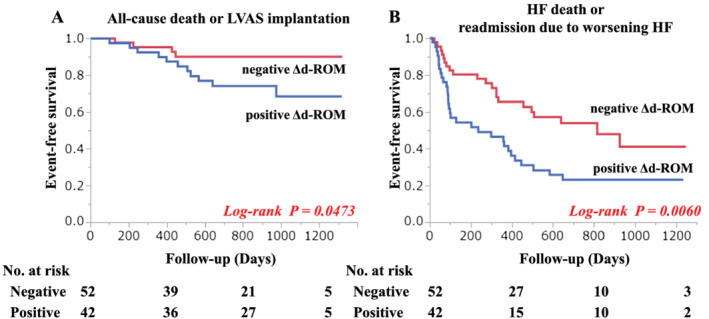
Kaplan–Meier estimates of risk of cardiac events.The patients whose Δd‐ROM was positive had a higher risk of all cause death or left ventricular assist system (LVAS) implantation than the patients whose Δd‐ROM is negative (log‐rank test P = 0.047) (A). A similar trend was indicated for HF death or readmission due to worsening HF (log‐rank test P = 0.006) (B).
Figure 2.
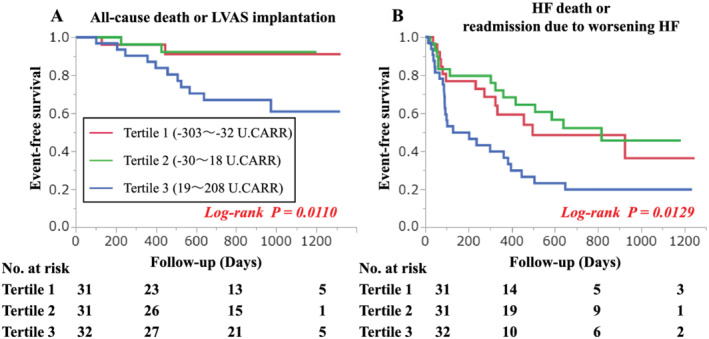
Kaplan–Meier estimates of risk of cardiac events according to tertiles of levels of Δd‐ROM.The Δd‐ROM levels were divided into tertiles. Kaplan–Meier analysis revealed that there were many cardiac events in the highest Δd‐ROM tertile compared with the lower two tertiles of Δd‐ROM levels. (A) Cardiac events were all cause death or LVAS implantation (log‐rank test P = 0.011). (B) Cardiac events were HF death or readmission due to worsening HF (log‐rank test P = 0.013).
Univariate Cox regression analysis showed that BMI, resting systolic and diastolic blood pressure, E/e′ ratio, AT, peak VO2 and VE/VCO2 slope were significant predictors for all‐cause death or LVAS implantation (Table S2 ). Cox regression analysis revealed that the serum d‐ROM levels before CPX did not predict prognosis in patients with HF. The BNP value did not remain as a prognostic factor, possibly because HF with reduced EF (HFrEF) and HF with preserved EF (HFpEF) were mixed in this population. Among those found to be a predictor of this was peak VO2, an established risk marker. Importantly, elevated Δd‐ROM levels were independently associated with increased mortality risk within patients with HF (Tertile 3 vs. 1; HR: 4.55; 95% CI: 1.22–29.43; P = 0.022) (Tables 4 and S2 ). The number of statistically acceptable variables calculated from the number of events was judged to be three, including Δd‐ROM. Therefore, we conducted an analysis using a model that combines BMI and blood pressure, focusing on peak VO2. Multivariate analysis adjusted for BMI and peak VO2 showed that Δd‐ROM was an independent factor of all‐cause death or LVAS implantation (Tertile 3 vs. 1; HR: 4.57; 95% CI: 1.21–29.77; P = 0.022), and adjusted for diastolic blood pressure and peak VO2 showed similar result (Tertile 3 vs. 1; HR: 4.95; 95% CI: 1.23–33.43; P = 0.022) (Tables 4 and S2 ). Furthermore, focused on the CPX results, Δd‐ROM was found to be an independent factor of all‐cause death or LVAS implantation (Tertile 3 vs. 1; HR: 7.15; 95% CI: 1.65–53.64; P = 0.006) when adjusted with peak VO2 and VE/VCO2 slope (Tables 4 and S2 ).
Table 4.
Univariate and multivariate analyses for all‐cause death or left ventricular assist system implantation
| Univariate analysis | Multivariate analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||||||||
| HR | (95% CI) | P‐value | HR | (95% CI) | P‐value | HR | (95% CI) | P‐value | HR | (95% CI) | P‐value | |
| Δd‐ROM, U.CARR | 1.007 | (1.000–1.014) | 0.052 | |||||||||
| Δd‐ROM, U.CARR | ||||||||||||
| Tertile 1 (−303 to −32) | 1 | 1 | 1 | 1 | ||||||||
| Tertile 2 (−30–18) | 0.879 | (0.105–7.330) | 0.898 | 0.870 | (0.104–7.256) | 0.889 | 0.870 | (0.100–7.174) | 0.870 | 1.179 | (0.134–10.741) | 0.875 |
| Tertile 3 (19–208) | 4.554 | (1.222–29.435) | 0.022 | 4.568 | (1.209–29.772) | 0.023 | 4.948 | (1.225–33.429) | 0.023 | 7.145 | (1.654–53.640) | 0.006 |
Multivariate analysis. Forced Inclusion Model 1 was adjusted for BMI and peakVO2. Forced Inclusion Model 2 was adjusted for diastolic blood pressure and peakVO2. Forced Inclusion Model 3 was adjusted for peakVO2 and VE/VCO2 slope.
CI, confidence interval; HR, hazard ratio.
Discussion
The key findings of our study were that (1) patients with HF were exposed to oxidative stress even at rest and (2) patients with HF whose oxidative stress was further enhanced by symptom‐limited exercise had a higher risk of adverse events. To the best of our knowledge, this is the first report to demonstrate the apparent relationship between acute exercise‐induced oxidative stress changes and clinical outcome in patients with HF.
Several reports previously showed the utility of measuring d‐ROM in patients with HF. Hirata et al. 20 noted that serum d‐ROM levels were correlated with HF severity as evaluated by NYHA functional class in patents with HFpEF where there were significantly more readmissions for worsening HF in the high d‐ROM group compared with the low d‐ROM group. However, serum d‐ROM levels before CPX were not correlated with cardiac events in the present study. This finding may be because of the different backgrounds in the patient population. In addition, the aetiology of HF was diverse in this study cohort; therefore, further research focusing on specific aetiologies is needed. If the aetiology is limited, fluctuations in oxidative stress due to exercise may have different consequences to those in this study.
In the present study, we demonstrated that increased oxidative stress in response to symptom‐limited exercise was associated with cardiac events in patients with HF, but the mechanism, including the source of ROS, is still unknown. Thus, several pathways can be cited as sources, that is, (1) production of ROS in mitochondria, (2) production of ROS associated with ischaemia reperfusion by blood redistribution and (3) production of ROS associated with inflammation of skeletal muscle injured by exercise, among other pathways. 25 We measured the blood metabolites produced by ROS in this study, but it could not be clearly shown which production pathway for oxidative stress was affected. In addition, the d‐ROM value mainly reflects ROOH, but not the entire ROS; therefore, the results of this study should be interpreted carefully. Because repeatability and reliability of reactive oxygen metabolites are at present weak, we also should be pay attention to the interpretation of ROS measurement results. The measurement system (FREE Carpe Diem; Wismerll, Tokyo, Japan) that we used in this study has been adopted in many previous literatures. In addition, our institution has published research papers that evaluated d‐ROM n patients after cardiac surgery using a same measurement system. 26 Although the patients' characteristics were different from our present study, the previous report and present study show similar tendencies of serum d‐ROM levels. Based upon these data, we believe that the accuracy of d‐ROM measurement is guaranteed. In addition, for the measurement of d‐ROM, analytes were measured in duplicate in all cases and adopted the average value in the present study. We consider that their repeatability and accuracy are guaranteed.
ROS is involved in energy metabolism, protein synthesis and mitochondrial biogenesis in the hormesis effects 27 ; therefore, the mild increase in oxidative stress associated with exercise is believed to have good effects on the human body. However, the failing myocardium has been reported to be unable to deal with the excess ROS, partially because of the reduction in the mtDNA copy number. 28 Thus, the increased oxidative stress marker in response to acute exercise may reflect inappropriate processing of excess oxidative stress in the failing heart. Our data suggest that if antioxidant capacity increases with exercise, the exercise will be beneficial, but if oxidative stress increases further with exercise, the exercise may be disadvantageous in patients with HF. However, the controversy about the level and format of exercise that can yield optimal beneficial effects in patients with HF continues in the literature. Based upon the present findings, we propose that measuring d‐ROM before and after acute exercise could be useful to determine the optimal exercise intensity. We believe that by prescribing exercise with an intensity that does not increase oxidative stress, it will be possible to perform highly individualized exercise therapy than before.
Limitations
This study has several limitations. First, this study was performed in a small number of cases in a single centre; thus, multicentre prospective trials are required to further improve the objectivity of our findings. Second, 30% (n = 28) of all cases in this study were HFpEF. Because HFpEF and HFrEF have molecularly and functionally different features, 29 , 30 it may be better to evaluate these conditions separately. In addition, many aetiologies were considered to be involved in this study, not only HFrEF or HFpEF. In fact, 17% of patients had ischaemic heart disease, 38% had atrial fibrillation, and about half had a history of hypertension. The number of patients in this study was small, and it was not possible to make adjustments for all aetiologies. Third, in this study, ischaemic cardiomyopathy accounted for only 17% of cases and may differ from the general HF population. In addition, biventricular pacing accounted for only 3% of cases, and those that were relatively mild may have been included in the overall cohort. Fourth, it was not possible to perform CPX at follow‐up and evaluate the changes in serum d‐ROM levels in response to acute exercise. Furthermore, follow‐up evaluations of cardiac function by echocardiography were insufficient; therefore, it was not possible to clarify whether the increase in oxidative stress accompanying exercise was prolonged or how it was involved in myocardial remodelling. Finally, it is difficult to evaluate cost performance in this study.
Conclusions
In conclusion, patients with HF showing an increased oxidative stress marker during a symptom‐limited exercise stress test had a poor prognosis. By judging the increase or decrease in oxidative stress in response to acute exercise, appropriate exercise methods and intensity can be determined for patients with HF.
Conflict of interest
None declared.
Funding
This work was supported by Grants‐in‐Aid for Scientific Research (C‐19K08493) to Y.I. and (C‐24591066) to M.Y.
Supporting information
Figure S1. Kaplan–Meier curves for all cause death. Kaplan–Meier analysis revealed that there were many all cause death in the highest Δd‐ROM tertile compared to the lower 2 tertiles of Δd‐ROM levels (log‐rank test P = 0.023).
Table S1. Single regression analysis between serum d‐ROM levels of before CPX and each continuous variable.
Table S2. Univariate and multivariate analyses for all‐cause death or left ventricular assist system implantation.
Acknowledgements
Serum d‐ROM level analysis was performed in the Research Support Platform of Osaka City University Graduate School of Medicine.
Shibata, A. , Izumiya, Y. , Yamaguchi, Y. , Kitada, R. , Iwata, S. , Ehara, S. , Izumi, Y. , Hanatani, A. , and Yoshiyama, M. (2021) Increased oxidative stress during exercise predicts poor prognosis in patients with acute decompensated heart failure. ESC Heart Failure, 8: 3885–3893. 10.1002/ehf2.13538.
References
- 1. Roger VL. Epidemiology of heart failure. Circ Res 2013; 113: 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C, Stroke Statistics S . Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation 2017; 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krumholz HM, Normand SL, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999‐2011. Circulation 2014; 130: 966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991; 83: 778–786. [DOI] [PubMed] [Google Scholar]
- 5. Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA, American Heart Association Exercise CR, Prevention Committee of the Council on Clinical Cardiology CoNPA, Metabolism CoC, Stroke N, Council on E, Prevention . Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 2013; 128: 873–934. [DOI] [PubMed] [Google Scholar]
- 6. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 7. Hambrecht R, Niebauer J, Fiehn E, Kalberer B, Offner B, Hauer K, Riede U, Schlierf G, Kubler W, Schuler G. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol 1995; 25: 1239–1249. [DOI] [PubMed] [Google Scholar]
- 8. Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 2007; 115: 3086–3094. [DOI] [PubMed] [Google Scholar]
- 9. Spinale FG, Coker ML, Thomas CV, Walker JD, Mukherjee R, Hebbar L. Time‐dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res 1998; 82: 482–495. [DOI] [PubMed] [Google Scholar]
- 10. McCord JM. Oxygen‐derived free radicals in postischemic tissue injury. N Engl J Med 1985; 312: 159–163. [DOI] [PubMed] [Google Scholar]
- 11. McMurray J, Chopra M, Abdullah I, Smith WE, Dargie HJ. Evidence of oxidative stress in chronic heart failure in humans. Eur Heart J 1993; 14: 1493–1498. [DOI] [PubMed] [Google Scholar]
- 12. Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, Jeejeebhoy KN. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol 1998; 31: 1352–1356. [DOI] [PubMed] [Google Scholar]
- 13. Ludovico P, Burhans WC. Reactive oxygen species, ageing and the hormesis police. FEMS Yeast Res 2014; 14: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8‐iso‐prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation 1998; 97: 1536–1539. [DOI] [PubMed] [Google Scholar]
- 15. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 16. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986; 60: 2020–2027. [DOI] [PubMed] [Google Scholar]
- 17. Kanzaki M, Asano Y, Ishibashi‐Ueda H, Oiki E, Nishida T, Asanuma H, Kato H, Oka T, Ohtani T, Tsukamoto O, Higo S, Kioka H, Matsuoka K, Sawa Y, Komuro I, Kitakaze M, Takashima S, Sakata Y. A development of nucleic chromatin measurements as a new prognostic marker for severe chronic heart failure. PLoS One 2016; 11: e0148209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV, American Heart Association Exercise CR, Prevention Committee of the Council on Clinical C, Council on E, Prevention, Council on Peripheral Vascular D, Interdisciplinary Council on Quality of C, Outcomes R . Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 2010; 122: 191–225. [DOI] [PubMed] [Google Scholar]
- 19. Palmieri B, Sblendorio V. Oxidative stress tests: overview on reliability and use. Part I. Eur Rev Med Pharmacol Sci 2007; 11: 309–342. [PubMed] [Google Scholar]
- 20. Hirata Y, Yamamoto E, Tokitsu T, Kusaka H, Fujisue K, Kurokawa H, Sugamura K, Maeda H, Tsujita K, Yamamuro M, Kaikita K, Hokimoto S, Sugiyama S, Ogawa H. Reactive oxidative metabolites are associated with the severity of heart failure and predict future cardiovascular events in heart failure with preserved left ventricular ejection fraction. Int J Cardiol 2015; 179: 305–308. [DOI] [PubMed] [Google Scholar]
- 21. Cesarone MR, Belcaro G, Carratelli M, Cornelli U, De Sanctis MT, Incandela L, Barsotti A, Terranova R, Nicolaides A. A simple test to monitor oxidative stress. Int Angiol 1999; 18: 127–130. [PubMed] [Google Scholar]
- 22. Cornelli U, Terranova R, Luca S, Cornelli M, Alberti A. Bioavailability and antioxidant activity of some food supplements in men and women using the D‐Roms test as a marker of oxidative stress. J Nutr 2001; 131: 3208–3211. [DOI] [PubMed] [Google Scholar]
- 23. Iamele L, Fiocchi R, Vernocchi A. Evaluation of an automated spectrophotometric assay for reactive oxygen metabolites in serum. Clin Chem Lab Med 2002; 40: 673–676. [DOI] [PubMed] [Google Scholar]
- 24. Chase PJ, Kenjale A, Cahalin LP, Arena R, Davis PG, Myers J, Guazzi M, Forman DE, Ashley E, Peberdy MA, West E, Kelly CT, Bensimhon DR. Effects of respiratory exchange ratio on the prognostic value of peak oxygen consumption and ventilatory efficiency in patients with systolic heart failure. JACC Heart Fail 2013; 1: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Banerjee AK, Mandal A, Chanda D, Chakraborti S. Oxidant, antioxidant and physical exercise. Mol Cell Biochem 2003; 253: 307–312. [DOI] [PubMed] [Google Scholar]
- 26. Suehiro K, Tanaka K, Matsuura T, Funao T, Yamada T, Mori T, Tsuchiya M, Nishikawa K. Preoperative hydroperoxide concentrations are associated with a risk of postoperative complications after cardiac surgery. Anaesth Intensive Care 2014; 42: 487–494. [DOI] [PubMed] [Google Scholar]
- 27. Martins I, Galluzzi L, Kroemer G. Hormesis, cell death and aging. Aging (Albany NY) 2011; 3: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res 1999; 85: 357–363. [DOI] [PubMed] [Google Scholar]
- 29. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 30. Komajda M, Lam CS. Heart failure with preserved ejection fraction: a clinical dilemma. Eur Heart J 2014; 35: 1022–1032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan–Meier curves for all cause death. Kaplan–Meier analysis revealed that there were many all cause death in the highest Δd‐ROM tertile compared to the lower 2 tertiles of Δd‐ROM levels (log‐rank test P = 0.023).
Table S1. Single regression analysis between serum d‐ROM levels of before CPX and each continuous variable.
Table S2. Univariate and multivariate analyses for all‐cause death or left ventricular assist system implantation.


