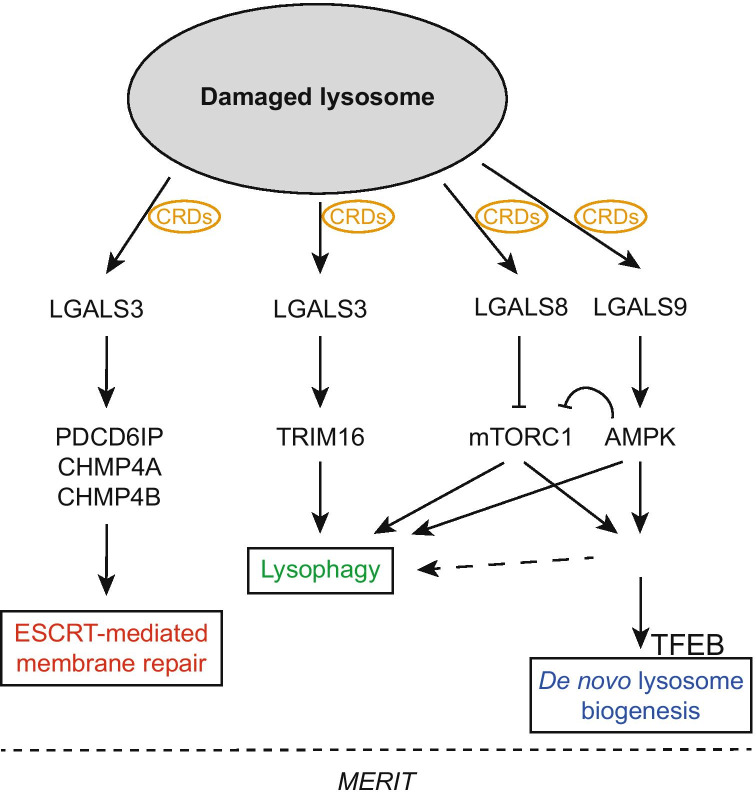
Fig. 5.
Model illustrating the membrane repair, removal and replacement (MERIT) system. Lysosome membrane damage leads to the cytoplasmic exposure of otherwise not accessible determinants for the cytoplasmic membrane integrity surveillance system. In particular, exposed N-glycans are a signal for danger and locally recruit and probably concentrate galectins (LGALS3, 8, 9), which bind the glycans through their carbohydrate recognition domains (CRDs). The bifunctionality of galectins allows them to engage downstream players of the MERIT system, which coordinates lysosomal membrane repair, lysosome removal and replacement. LGALS3 recruits the endosomal sorting complexes required for transport (ESCRT) components programmed cell death 6 interacting protein (PDCD6IP), charged multivesicular body protein 4A (CHMP4A) and CHMP4B, and promotes the formation of ESCRT machinery to repair the damaged lysosomal membranes. LGALS3 also cooperates with tripartite motif containing 16 (TRIM16) to guide autophagy initiation machinery to turnover terminally injured lysosomes. Synergistically, LGALS8 inactivates target of rapamycin complex 1 (mTORC1), while LGALS9 activates AMP-activated kinase (AMPK), which further inhibits mTORC1. The mTORC1 inactivation and AMPK activation lead to cell growth arrest, induction of autophagy and transcription factor EB (TFEB) nuclear translocation to replace damaged lysosomes through de novo biogenesis