Abstract
Aims
Although the reno‐protective effects of sodium–glucose cotransporter 2 inhibitors are known in patients with heart failure or type 2 diabetes mellitus (T2DM), this effect has not been confirmed in patients with acute myocardial infarction (AMI).
Methods and results
The prospective, multicentre, randomized, double‐blind, placebo‐controlled EMBODY trial investigated patients with AMI and T2DM in Japan. The eligible patients included adults aged 20 years or older, diagnosed with AMI and T2DM, and who could be discharged within 2–12 weeks after the onset of AMI. One hundred and five patients were randomized (1:1) to receive once daily 10 mg empagliflozin or placebo within 2 weeks of AMI onset. In this sub‐analysis, we investigated the time course of renal functional parameters such as serum creatinine levels and estimated glomerular filtration rate (eGFR) from baseline to Weeks 4, 12, and 24. Ninety‐six patients (64 ± 11 years, 78 male) were included in the full analysis (n = 46 and 50 in the empagliflozin and placebo groups, respectively). We used serum creatinine and eGFR as indicators of renal function. In the placebo group, eGFR decreased from 66.14 mL/min/1.73 m2 at baseline to 62.77 mL/min/1.73 m2 by Week 24 (P = 0.023) but remained unchanged in the empagliflozin group (from 64.60 to 64.36 mL/min/1.73 m2, P = 0.843). In the latter group, uric acid improved from 5.8 mg/dL at baseline to 4.9 mg/dL at Week 24 (P < 0.001). In the earlier analysis of 56 patients with eGFR ≥ 60 mL/min/1.73 m2, the eGFR decreased and the serum creatinine increased from baseline to 24 weeks in the placebo group, significantly different to the empagliflozin group (−6.61 vs. +0.22 mL/min/1.73 m2, P = 0.008 and +0.063 vs. −0.001 mg/dL, P = 0.030, respectively). The changes in serum creatinine and eGFR from baseline to Week 24 were significantly correlated with those in uric acid in the placebo group (r = 0.664, P < 0.001 and r = −0.675, P < 0.001, respectively) but not in the empagliflozin group.
Conclusions
Empagliflozin prevented the kidney functional decline in patients with AMI and T2DM, especially those with baseline eGFR ≥ 60 mL/min/1.73 m2. Early administration of sodium–glucose cotransporter 2 inhibitors in these patients is considered desirable for renal protection.
Keywords: Acute myocardial infarction, Empagliflozin, Estimated glomerular filtration rate, Sodium–glucose cotransporter 2 inhibitor, Type 2 diabetes mellitus, Uric acid
Introduction
Type 2 diabetes mellitus (T2DM) is one of the most common diseases and the leading cause of end‐stage renal and cardiovascular (CV) disease 1 ; it has emerged as a social problem worldwide. 2 Sodium–glucose cotransporter 2 (SGLT2) inhibitors, released in the 2010s as a new class of glucose lowering drugs, reduce blood pressure (BP) and body weight (BW) in addition to improving blood glucose by urinary glucosuria. 3 SGLT2 inhibitors have been shown to be beneficial in the reduction of CV events and improving renal outcomes in recent, large, randomized, placebo‐controlled trials (EMPA‐REG OUTCOME trial, CANVAS trial, and DECLARE–TIMI 58 study). 4 , 5 , 6 The effects of SGLT2 inhibitors such as empagliflozin as osmotic diuretics and on natriuresis may underlie the CV and renal benefits demonstrated in the recent EMPA‐REG OUTCOME trial. 4 The reno‐protective effect of SGLT2 inhibitors was demonstrated in the recent CREDENCE and DAPA‐CKD trials. 7 , 8
Although reno‐protective effects of SGLT2 inhibitors have been recognized in patients with heart failure or T2DM, this protection has not been fully examined in patients with acute myocardial infarction (AMI). We therefore examined whether empagliflozin, an SGLT2 inhibitor, shows reno‐protective effects in patients with AMI and T2DM. This study was a sub‐analysis of the EMBODY trial, which showed that a decrease in cardiac sympathetic hyperactivity associated with empagliflozin may contribute to the prevention of CV events, including sudden cardiac death, in patients with T2DM and AMI treated with SGLT2 inhibitors in Japan. 9
Methods
Trial design
The EMBODY trial is a recently published prospective, multicentre, randomized, double‐blind, placebo‐controlled trial comprising patients with AMI and T2DM in Japan. 10 One hundred and five patients were randomized (1:1) to receive once daily placebo or empagliflozin (10 mg) within 2 weeks of AMI onset. In the present sub‐analysis, we specifically focused on the time course of renal function from baseline to Weeks 4, 12, and 24 after AMI. We used serum creatinine levels and estimated glomerular filtration rate (eGFR) as indicators of renal function.
Trial population and follow‐up
The trial recruitment period was from February 2018 to March 2019, and the last visit of the last patient was completed in August 2019. The inclusion and exclusion criteria have been published previously. 10 Once each patient had provided written informed consent for participation, been randomized, and assigned to either the empagliflozin or placebo group, the follow‐up visits were scheduled at 4, 12, and 24 weeks. Patients received standard treatment for their underlying diseases, T2DM, and AMI during the trial period. In this study, blood tests for all cases were performed on an empty stomach.
The left ventricular ejection fraction was calculated using the modified Simpson's method via transthoracic echocardiography. The tricuspid regurgitation velocity was obtained using continuous wave Doppler imaging in the right ventricular inflow or apical four‐chamber views. The trans‐tricuspid pressure gradient (TRPG) was calculated as follows:
The peak early diastolic phase (E) and late diastolic phase (A) mitral inflow velocities and the E/A ratio were measured using pulsed‐wave Doppler echocardiography with the sample volume between the mitral leaflet tips. The mitral annular velocity (E′) and mean E/E′ ratio were measured at the septal and lateral annuli using tissue Doppler imaging. The left atrial diameter was measured using B‐mode during systole in the parasternal long‐axis view.
Randomization and blinding
Patients with AMI and T2DM were randomly assigned to an empagliflozin (10 mg/day) group or a placebo group, both as add‐ons to conventional therapy within 2 weeks of the onset of AMI, based on allocation factors, baseline glycated haemoglobin (HbA1c) values (˂7.0% or ≥7.0%), and max creatine kinase levels (˂3000 or ≥3000 IU/L) using a dynamic allocation method. Post‐randomization follow‐up visits were scheduled at 4, 12, and 24 weeks. This study was double‐blinded. Patients also received post‐AMI treatment with beta‐blockers, anti‐platelet therapy, statins, and renin–angiotensin system inhibitors in accordance with local guidelines. 11 , 12 Throughout the trial, investigators were encouraged to treat other CV risk factors (including dyslipidaemia and hypertension) to ensure the best available standard of care to patients.
Trial endpoints
The primary endpoints of this study were changes in renal functional markers (eGFR, serum creatinine, cystatin, and urinary albumin) from baseline to 24 weeks. The secondary endpoints were the correlations between these renal functional markers and other factors [systolic BP (SBP), serum uric acid, haematocrit, and ketones].
Renal functional markers
Estimated glomerular filtration rate
The glomerular filtration rate (GFR) is a key indicator of renal function. The eGFR is a mathematically derived value, based on a patient's serum creatinine level, age, sex, and race. Although the Modification of Diet in Renal Disease 13 and Chronic Kidney Disease Epidemiology Collaboration 14 equations are used internationally, the GFR estimation formula for the Japanese population based on the serum creatinine value was used for eGFR calculations in this study, as the former tends to overestimate renal function in Japanese individuals. 15 The eGFR estimation formula used in this study is shown as follows:
For women, the obtained value is multiplied by 0.739.
Chronic kidney disease stage
According to the Kidney Disease Outcome Quality Initiative 16 and Kidney Disease: Improving Global Outcome 13 guidelines, patients were defined as having chronic kidney disease (CKD) if they had abnormalities of kidney function or structure for more than 3 months. Kidney disease severity is classified into five stages according to the eGFR level. In this study, patients were classified for analysis as follows: eGFR ≥ 60 mL/min/1.73 m2, which indicated normal kidney function to mild renal impairment (CKD Stage 1 or 2), and eGFR between 45 and 59 mL/min/1.73 m2, which corresponded to early renal insufficiency (CKD Stage 3).
Statistical analyses
All continuous values and categorical variables are expressed as mean ± standard deviation and the number and percentage of patients, respectively. Mixed‐effects model repeated measures analysis was used to compare changes in primary outcomes: BP, BW, HbA1c, eGFR, haematocrit, uric acid, glycaemic and lipid parameters, and serum ketone bodies, from baseline to 24 weeks. For other outcomes, a t‐test was used to analyse continuous variables and the Wilcoxon signed‐rank test was used for categorical variables. A two‐sided probability value of P < 0.05 was considered statistically significant. In addition to the earlier analysis, the cohort was stratified into two groups based on baseline eGFR [45 mL/min/1.73 m2 ≤ eGFR < 60 mL/min/1.73 m2 (eGFR 45–59) and eGFR ≥ 60 mL/min/1.73 m2 (eGFR ≥ 60)] for subgroup analysis. All statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC, USA).
Ethics approval and consent to participate
The EMBODY trial was registered at the UMIN in November 2017 (ID: 000030158). The local institutional review boards and independent ethics committees approved the trial protocol. The investigation conformed with the principles outlined in the Declaration of Helsinki and was performed according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects established by the Ministry of Health, Labour, and Welfare and the Ministry of Education, Culture, Sports, Science, and Technology in Japan. After initial screening for eligibility using prior medical records, each patient received an adequate explanation of the trial plan before they provided written informed consent.
Results
A total of 105 AMI patients with T2DM met the inclusion criteria, consented to participate in the trial between February 2018 and March 2019, and were randomized into two groups. Six patients in the empagliflozin group and three in the placebo group withdrew their consent and were thus excluded before medication was initiated. Therefore, 96 patients were finally included in the full analysis (46 in the empagliflozin group and 50 in the placebo group) (Figure 1). Baseline characteristics were not significantly different between the treatment groups (Table 1).
Figure 1.
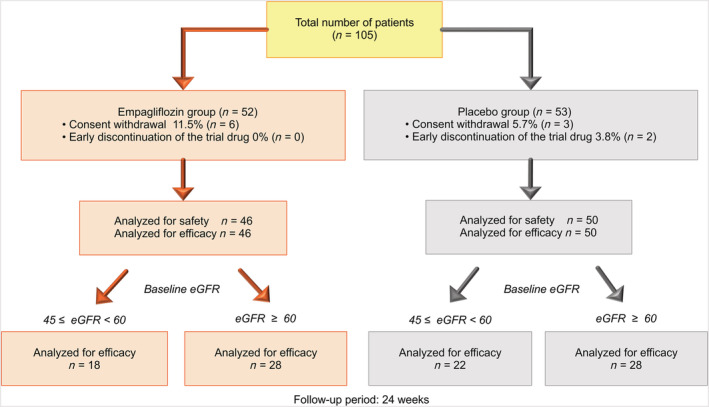
Flow chart of the study patients. A total of 105 patients met the inclusion criteria and were randomized in this study. Six patients in the empagliflozin group and three patients in the placebo group were excluded because of consent withdrawal before medication began. Therefore, 96 patients were included in the final analysis (46 in the empagliflozin group and 50 in the placebo group). Additional stratified analysis was performed according to baseline estimated glomerular filtration rate (eGFR).
Table 1.
Clinical characteristics of the study population
| Variable | Empagliflozin | Placebo | P value |
|---|---|---|---|
| n = 46 | n = 50 | ||
| Men, n (SD) | 38 (82.6) | 39 (78.0) | 0.616 |
| Age, years (SD) | 63.9 (10.4) | 64.6 (11.6) | 0.734 |
| Body weight, kg (SD) | 70.1 (13.7) | 68.1 (14.4) | 0.493 |
| BMI, kg/m2 (SD) | 25.2 (3.7) | 25.2 (4.1) | 0.992 |
| DM duration, months (SD) | 38.3 (43.4) | 32.4 (43.3) | 0.507 |
| Current smoker, n (%) | 24 (52.2) | 27 (54.0) | 0.913 |
| Medical history | |||
| Cerebrocardiovascular disease, n (%) | 7 (15.2) | 11 (22.0) | 0.442 |
| Hypertension, n (%) | 38 (82.6) | 39 (78.0) | 0.617 |
| Dyslipidaemia, n (%) | 34 (73.9) | 36 (72.0) | 1.000 |
| NYHA classification | |||
| I/II, % | 92.6/7.4 | 83.9/16.1 | 0.309 |
| Killip's classification at admission | |||
| I/II, % | 77.8/7.4 | 58.1/22.6 | 0.087 |
| III/IV, % | 11.1/3.7 | 9.7/9.7 | 0.429 |
| Contrast medium usage, mL (SD) | 157.4 (50.4) | 147.8 (74.0) | 0.582 |
| Blood sampling test | |||
| Max CK, IU/L (SD) | 2080.7 (2461.6) | 2358.7 (2829.1) | 0.610 |
| HbA1c, % (SD) | 6.82 (1.00) | 6.89 (0.92) | 0.735 |
| Uric acid, mg/dL (SD) | 5.8 (1.4) | 5.7 (1.5) | 0.935 |
| Creatinine, mg/dL (SD) | 0.922 (0.19) | 0.887 (0.20) | 0.392 |
| eGFR, mL/min/1.73 m2 (SD) | 64.60 (14.95) | 66.14 (15.72) | 0.624 |
| Urinary albumin amount, mg/g Cr (SD) | 130.88 (333.8) | 56.65 (112.3) | 0.181 |
| Cystatin C, mg/L (SD) | 1.132 (0.27) | 1.110 (0.34) | 0.784 |
| Haematocrit, % (SD) | 40.5 (4.6) | 40.3 (4.2) | 0.858 |
| NT‐proBNP, pg/mL (SD) | 1028.7 (1105.6) | 1270.6 (1521.0) | 0.450 |
| Transthoracic echocardiography | |||
| LVEF, % (SD) | 55.13 (14.19) | 55.01 (13.65) | 0.972 |
| E/A (SD) | 0.94 (0.49) | 1.01 (0.69) | 0.645 |
| Average E/E′ (SD) | 13.47 (7.24) | 13.73 (6.26) | 0.883 |
| Left atrial dimension, mm (SD) | 36.36 (8.61) | 36.71 (5.56) | 0.854 |
| TRPG, mmHg (SD) | 17.59 (7.48) | 20.71 (11.49) | 0.234 |
| Medical therapy | |||
| Beta‐blocker, n (%) | 41 (89.1) | 38 (76.0) | 0.11 |
| ARB, n (%) | 22 (47.8) | 19 (38.0) | 0.41 |
| ACE‐I, n (%) | 23 (50.0) | 28 (56.0) | 0.68 |
| Statin, n (%) | 44 (95.7) | 48 (96.0) | 1.00 |
| Spironolactone, n (%) | 11 (23.9) | 12 (24.0) | 1.00 |
| Diuretics, n (%) | 8 (17.4) | 11 (22.0) | 0.62 |
| Metformin, n (%) | 7 (15.2) | 6 (12.0) | 0.77 |
| DPP‐4 inhibitor, n (%) | 20 (43.5) | 23 (46.0) | 0.84 |
| ASA/P2Y12 inhibitor, n (%) | 46 (100) | 50 (100) | 1.00 |
| DOAC, n (%) | 3 (6.5) | 3 (6.0) | 1.00 |
| NSAIDs, n (%) | 4 (8.7) | 2 (4.0) | 0.35 |
A, late diastolic phase mitral inflow velocity; ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; BMI, body mass index; CK, creatine kinase; DM, diabetes mellitus; DOAC, direct oral anticoagulant; DPP‐4, dipeptidyl peptidase‐4; E, peak early diastolic phase mitral inflow velocity; E′, mitral annular velocity; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; LVEF, left ventricular ejection fraction; NSAIDs, non‐steroidal anti‐inflammatory drugs; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation; TRPG, trans‐tricuspid pressure gradient.
In terms of changes in parameters from baseline to 24 weeks, SBP and BW were decreased in the empagliflozin group and increased in the placebo group, a significant difference between the groups (−6.6 ± 14.3 vs. 3.5 ± 18.6 mmHg, P = 0.004 and −2.23 ± 3.56 vs. 0.08 ± 2.25 kg, P = 0.001, respectively). Intergroup comparison showed that there was no significant difference between the treatment groups in terms of the amount of change in the serum creatinine levels and eGFR from baseline to 24 weeks (P = 0.236 and P = 0.120, respectively) (Figure 2). Although intra‐group comparison showed that the creatinine values and eGFR had not changed significantly from baseline to 24 weeks in the empagliflozin group (0.922–0.937 mg/dL, P = 0.456 and 64.60–64.36 mL/min/1.73 m2, P = 0.843, respectively), both parameters significantly worsened in the placebo group (0.887–0.935 mg/dL, P = 0.008 and 66.14–62.77 mL/min/1.73 m2, P = 0.023, respectively). Intra‐group and intergroup comparisons showed no significant differences in the change in cystatin C and urinary albumin concentrations from baseline to 24 weeks (Figure 2). Changes in other blood parameters from baseline to 24 weeks are shown in Table 2. Intergroup comparison showed that changes in serum uric acid and haematocrit levels from baseline to 24 weeks were significantly different between the empagliflozin group and the placebo group (−0.89 ± 1.11 vs. 0.03 ± 1.03 mg/dL, P < 0.001 and 3.70 ± 3.72% vs. 0.16 ± 4.52%, P < 0.001, respectively).
Figure 2.
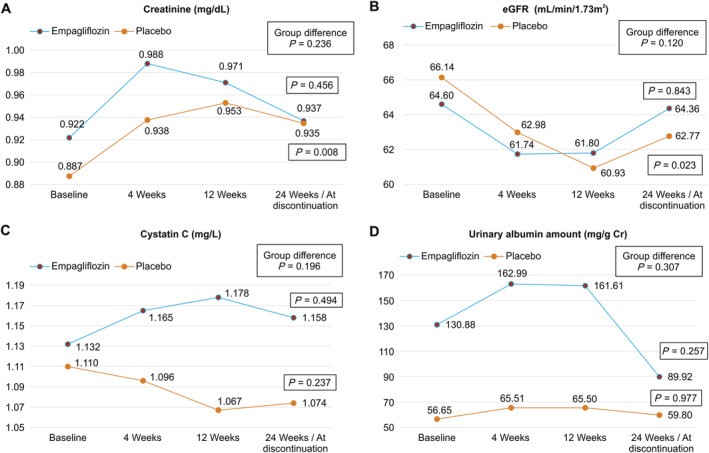
Changes from baseline in (A) creatinine, (B) estimated glomerular filtration rate (eGFR), (C) cystatin C, and (D) urinary albumin. In the analysis within each group, though changes in creatinine values and eGFR at 24 weeks compared with those in baseline values were not significantly different in the empagliflozin group, both were significantly worse at 24 weeks in the placebo group.
Table 2.
Comparison of changes in parameters between the two treatment groups
| Empagliflozin group | Placebo group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Average | SD | P value a | n | Average | SD | P value a | P value b | |
| SBP (mmHg) | |||||||||
| Baseline | 46 | 129.7 | 11.9 | 50 | 123.1 | 15.7 | |||
| 24 weeks | 46 | 123.1 | 12.7 | 49 | 126.2 | 17.8 | |||
| Changes | 46 | −6.6 | 14.3 | 0.003 | 49 | 3.5 | 18.6 | 0.190 | 0.004 |
| DBP (mmHg) | |||||||||
| Baseline | 46 | 75.3 | 9.2 | 50 | 71.9 | 9.9 | |||
| 24 weeks | 46 | 73. | 9.1 | 49 | 73.7 | 11.3 | |||
| Changes | 46 | −1.5 | 10.9 | 0.343 | 49 | 1.8 | 13.3 | 0.360 | 0.191 |
| BW (kg) | |||||||||
| Baseline | 46 | 70.08 | 13.74 | 50 | 68.10 | 14.40 | |||
| 24 weeks | 46 | 67.85 | 12.58 | 50 | 68.19 | 14.16 | |||
| Changes | 46 | −2.23 | 3.56 | <0.001 | 50 | 0.08 | 2.25 | 0.836 | 0.001 |
| BMI (kg/m2) | |||||||||
| Baseline | 46 | 25.18 | 3.69 | 50 | 25.17 | 4.07 | |||
| 24 weeks | 46 | 24.39 | 3.39 | 50 | 25.21 | 4.04 | |||
| Changes | 46 | −0.78 | 1.22 | <0.001 | 50 | 0.04 | 1.12 | 0.809 | <0.001 |
| RBC (× 104/μL) | |||||||||
| Baseline | 46 | 449.9 | 52.8 | 50 | 439.6 | 52.7 | |||
| 24 weeks | 46 | 492.8 | 45.6 | 50 | 444.7 | 56.6 | |||
| Changes | 46 | 42.9 | 46.8 | <0.001 | 50 | 5.1 | 54.9 | 0.513 | <0.001 |
| WBC (/μL) | |||||||||
| Baseline | 46 | 6679.8 | 2062.8 | 50 | 7173 | 2029.7 | |||
| 24 weeks | 46 | 6394.6 | 1654.3 | 50 | 6658.8 | 1338.1 | |||
| Changes | 46 | −285.2 | 1857.2 | 0.303 | 50 | −514.2 | 1818.3 | 0.051 | 0.543 |
| Hb (g/dL) | |||||||||
| Baseline | 46 | 13.53 | 1.85 | 50 | 13.58 | 1.48 | |||
| 24 weeks | 46 | 14.69 | 1.52 | 50 | 13.66 | 1.58 | |||
| Changes | 46 | 1.16 | 1.31 | <0.001 | 50 | 0.08 | 1.54 | 0.702 | <0.001 |
| Ht (%) | |||||||||
| Baseline | 46 | 40.50 | 4.55 | 50 | 40.34 | 4.21 | |||
| 24 weeks | 46 | 44.20 | 3.86 | 50 | 40.50 | 4.22 | |||
| Changes | 46 | 3.70 | 3.72 | <0.001 | 50 | 0.16 | 4.52 | 0.806 | <0.001 |
| Plt (×104/μL) | |||||||||
| Baseline | 46 | 25.11 | 9.92 | 50 | 23.30 | 6.81 | |||
| 24 weeks | 46 | 23.55 | 9.74 | 50 | 21.10 | 4.83 | |||
| Changes | 46 | −1.57 | 5.53 | 0.061 | 50 | −2.21 | 5.96 | 0.012 | 0.586 |
| AST (U/L) | |||||||||
| Baseline | 46 | 24.7 | 7.9 | 50 | 24.4 | 10.3 | |||
| 24 weeks | 46 | 23.2 | 8.5 | 50 | 25.4 | 13.9 | |||
| Changes | 46 | −1.5 | 9.6 | 0.280 | 50 | 1.0 | 14.8 | 0.629 | 0.322 |
| ALT (U/L) | |||||||||
| Baseline | 46 | 26.4 | 14.8 | 50 | 26.6 | 16.4 | |||
| 24 weeks | 46 | 22.3 | 13.1 | 50 | 24.2 | 11.9 | |||
| Changes | 46 | −4.1 | 12.9 | 0.039 | 50 | −2.4 | 16.9 | 0.320 | 0.594 |
| γ‐GTP (U/L) | |||||||||
| Baseline | 46 | 39.6 | 29.1 | 50 | 32.4 | 18.9 | |||
| 24 weeks | 46 | 40.0 | 40.2 | 50 | 39.2 | 38.1 | |||
| Changes | 46 | 0.3 | 28.3 | 0.934 | 50 | 6.8 | 31.9 | 0.136 | 0.296 |
| Uric acid (mg/dL) | |||||||||
| Baseline | 46 | 5.75 | 1.43 | 50 | 5.73 | 1.46 | |||
| 24 weeks | 46 | 4.86 | 1.35 | 49 | 5.76 | 1.45 | |||
| Changes | 46 | −0.89 | 1.11 | <0.001 | 49 | 0.03 | 1.03 | 0.815 | <0.001 |
| Creatinine (mg/dL) | |||||||||
| Baseline | 46 | 0.922 | 0.187 | 50 | 0.887 | 0.204 | |||
| 24 weeks | 46 | 0.937 | 0.245 | 50 | 0.935 | 0.214 | |||
| Changes | 46 | 0.015 | 0.135 | 0.456 | 50 | 0.047 | 0.121 | 0.008 | 0.236 |
| eGFR (mL/min/1.73 m2) | |||||||||
| Baseline | 46 | 64.60 | 14.95 | 50 | 66.14 | 15.72 | |||
| 24 weeks | 46 | 64.36 | 16.84 | 50 | 62.77 | 15.44 | |||
| Changes | 46 | −0.24 | 8.19 | 0.843 | 50 | −3.37 | 10.12 | 0.023 | 0.120 |
| NT‐proBNP (pg/mL) | |||||||||
| Baseline | 33 | 1028.7 | 1105.6 | 36 | 1270.6 | 1521.2 | |||
| 24 weeks | 32 | 370.3 | 530.9 | 33 | 673.7 | 1151.1 | |||
| Changes | 32 | −633.6 | 822.9 | <0.001 | 33 | −603.9 | 1180.1 | 0.006 | 0.907 |
| Blood ketone bodies (μmol/L) | |||||||||
| Baseline | 29 | 119.8 | 187.9 | 33 | 62.1 | 47.2 | |||
| 24 weeks | 32 | 119.3 | 169.2 | 33 | 61.2 | 35.7 | |||
| Changes | 32 | 49.7 | 175.4 | 0.120 | 33 | −16.6 | 73.5 | 0.203 | 0.055 |
| High‐sensitivity CRP (mg/dL) | |||||||||
| Baseline | 27 | 0.300 | 0.526 | 30 | 0.152 | 0.214 | |||
| 24 weeks | 27 | 0.175 | 0.208 | 30 | 0.093 | 0.160 | |||
| Changes | 27 | −0.126 | 0.564 | 0.257 | 30 | −0.059 | 0.211 | 0.138 | 0.547 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BW, body weight; CRP, C‐reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; Hb, haemoglobin; Ht, haematocrit; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; Plt, platelet; RBC, red blood cell; SBP, systolic blood pressure; SD, standard deviation; WBC, white blood cell; γ‐GTP, γ‐glutamyl transpeptidase.
Within‐group comparisons, 24 weeks compared with baseline.
Between‐group comparisons of changes over time.
Subgroup analyses in the estimated glomerular filtration rate 45–59 group
Baseline characteristics were not significantly different between the empagliflozin and placebo subgroups in the eGFR 45–59 group (67.8 ± 10.0 years, male 80%, and n = 18 in the empagliflozin subgroup and n = 22 in the placebo subgroup) (Supporting Information, Table S1). Although intra‐group and intergroup comparisons showed that there was no significant difference in the change in renal functional markers between these subgroups from baseline to 24 weeks (Figure 3A–3D), upon intra‐group comparison, the serum uric acid level had significantly decreased in the empagliflozin subgroup from baseline to 24 weeks (6.54 ± 1.34 to 5.47 ± 1.07 mg/dL, P = 0.014) but not in the placebo subgroup (5.93 ± 1.66 to 5.73 ± 1.48 mg/dL, P = 0.682). Upon intergroup comparison, the uric acid level decreased significantly more in the empagliflozin subgroup than in the placebo subgroup (−1.08 ± 0.94 vs. −0.20 ± 1.01 mg/dL, P = 0.009). Changes in other parameters studied in this sub‐analysis are shown in Supporting Information, Table S1.
Figure 3.
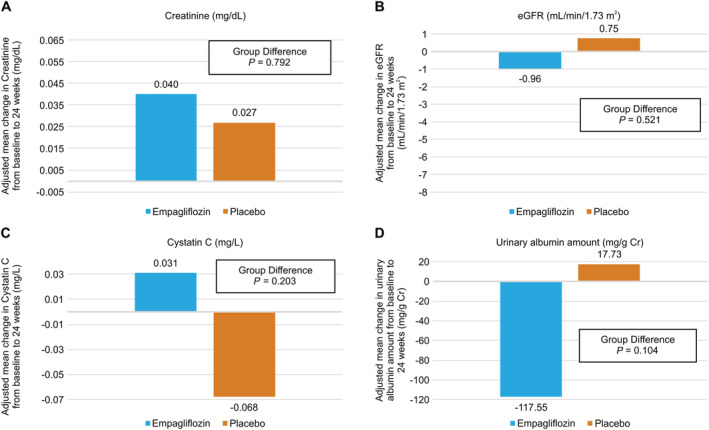
Amount of change in (A) creatinine, (B) estimated glomerular filtration rate (eGFR), (C) cystatin C, and (D) urinary albumin at 24 weeks from baseline in patients with baseline eGFR 45–59 and eGFR ≥ 60, respectively. In the eGFR 45–59 group, there was no significant difference between the two groups, but in the eGFR ≥ 60 group, creatinine and eGFR were maintained in the empagliflozin group but not in the placebo group, a significant difference.
Subgroup analyses in the estimated glomerular filtration rate ≥60 group
Supporting Information, Table S2 shows the baseline characteristics of patients in the eGFR ≥ 60 group (61.8 ± 10.9 years, male 80.4%, and n = 28 in the empagliflozin subgroup and n = 28 in the placebo subgroup). Baseline characteristics were not significantly different between the empagliflozin and placebo subgroups, except for cystatin C (1.02 ± 0.17 vs. 0.88 ± 0.15 mg/L, P = 0.039). In the placebo subgroup, the eGFR decreased and the serum creatinine increased from baseline to 24 weeks, significantly different compared with those in the empagliflozin subgroup (−6.61 vs. +0.22 mL/min/1.73 m2, P = 0.008 and +0.063 vs. −0.001 mg/dL, P = 0.030, respectively) (Figure 4A and 4B). Regarding the serum uric acid level, the same tendency as that of the eGFR 45–59 group was obtained; in the intra‐group comparison, the serum uric acid level had significantly decreased in the empagliflozin subgroup from baseline to 24 weeks (5.24 ± 1.21 to 4.47 ± 1.35 mg/dL, P = 0.031) but not in the placebo subgroup (5.57 ± 1.24 to 5.79 ± 1.40 mg/dL, P = 0.553), and in the intergroup comparison, the uric acid level decreased in the empagliflozin subgroup and increased in the placebo subgroup, a significant difference (−0.78 ± 1.17 vs. 0.23 ± 0.99 mg/dL, P = 0.001). Changes in other parameters in this sub‐analysis are shown in Supporting Information, Table S2.
Figure 4.
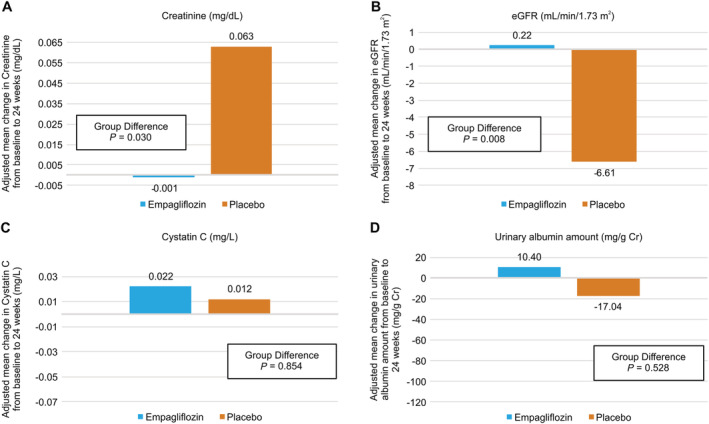
Amount of change in (A) creatinine, (B) estimated glomerular filtration rate (eGFR), (C) cystatin C, and (D) urinary albumin at 24 weeks from baseline in patients with baseline eGFR 45–59 and eGFR ≥ 60, respectively. In the eGFR 45–59 group, there was no significant difference between the two groups, but in the eGFR ≥ 60 group, creatinine and eGFR were maintained in the empagliflozin group but not in the placebo group, a significant difference.
Correlation between renal functional markers and various parameters
Table 3 shows correlation between the renal functional markers and various clinical parameters in the entire study population. In the entire study population, the changes in serum creatinine and eGFR from baseline to Week 24 were significantly correlated with those in uric acid (Figure 5B) and SBP in the placebo group, but not in the empagliflozin group (Figure 5A). In the eGFR ≥ 60 subgroup, the changes in serum creatinine and eGFR from baseline to Week 24 were also significantly correlated with those in uric acid (Figure 5D) and SBP in the placebo group but not in the empagliflozin group (Figure 5C and Supporting Information, Table S4). However, this was not the case in the eGFR 45–59 group (Figure 5E and 5F and Supporting Information, Table S3).
Table 3.
Correlation between renal functional markers and various parameters in the entire study population
| Creatinine (mg/dL) | eGFR (mL/min/1.73 m2) | ||||
|---|---|---|---|---|---|
| r | P value | r | P value | ||
| SBP (mmHg) | All cohort | −0.131 | 0.209 | 0.129 | 0.214 |
| Empagliflozin group | −0.001 | 0.993 | −0.090 | 0.558 | |
| Placebo group | −0.331 | 0.020 | 0.352 | 0.013 | |
| HbA1c (%) | All cohort | 0.051 | 0.623 | −0.011 | 0.911 |
| Empagliflozin group | −0.038 | 0.800 | 0.114 | 0.452 | |
| Placebo group | 0.139 | 0.335 | −0.098 | 0.500 | |
| Uric acid (mg/dL) | All cohort | 0.251 | 0.014 | −0.317 | 0.002 |
| Empagliflozin group | 0.077 | 0.610 | −0.079 | 0.603 | |
| Placebo group | 0.378 | 0.007 | −0.438 | 0.002 | |
eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; SBP, systolic blood pressure.
Figure 5.
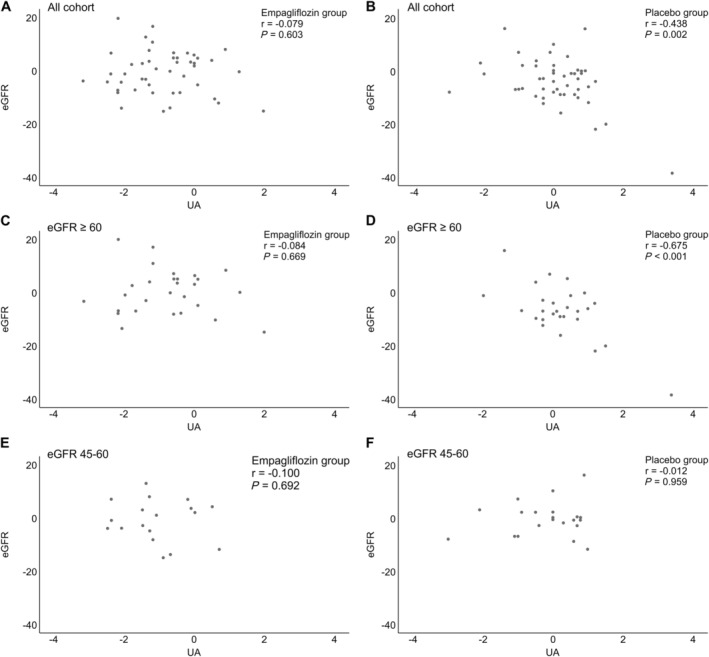
Correlation of the change from baseline to 24 weeks in the estimated glomerular filtration rate (eGFR) vs. that of serum uric acid levels in the empagliflozin (A, C, E) and placebo (B, D, F) subgroups. The placebo group showed a negative correlation between eGFR and uric acid levels, whereas no such correlation was observed in the empagliflozin group.
Discussion
In summary, for the sub‐analysis of the EMBODY trial in the entire study population, serum creatinine and eGFR at Week 24 did not change when compared with their baseline values in the empagliflozin group but were significantly worse over time in the placebo group. The serum uric acid level was significantly decreased in the empagliflozin group but remained unchanged in the placebo group. In the placebo group, there was a significant correlation between the deterioration of renal function and changes in uric acid levels and SBP. These tendencies were more pronounced in the eGFR ≥ 60 subgroup but not in the eGFR 45–59 subgroup. In previous studies, 7 , 8 decreases in the GFR in the acute phase after administration of SGLT2 inhibitors were observed. Such a decrease was also observed early on in this study, and the eGFR decreased in the first 4 weeks after administration of empagliflozin but increased thereafter and recovered to baseline eGFR levels at 24 weeks. In a previous study of Japanese subjects, 17 the eGFR was retained from 12 to 52 weeks after SGLT2 inhibitor administration despite an initial decrease, and a high eGFR at baseline was associated with a larger initial decrease, which is consistent with the results of this study. The DAPA‐CKD trial, 8 in contrast to this study, revealed a more sensitive renal protective effect of SGLT2 inhibitors in the group with a low baseline eGFR. A possible reason for the discrepancy is that, in this study, drugs were used from the acute stage after the onset of AMI and the follow‐up period was 24 weeks. If our follow‐up period was 72 weeks, as in the DAPA‐CKD trial, we may have discovered a renal protective effect even in patients with a low baseline eGFR. While the DAPA‐CKD trial was targeted at patients who were already taking renin–angiotensin–aldosterone system inhibitors, renin–angiotensin–aldosterone system inhibitors were introduced during hospitalization in about half of the cases in this study, which may also have affected the results.
Reno‐protective effects of empagliflozin in patients with acute myocardial infarction and type 2 diabetes mellitus
Although the protective effects of SGLT2 inhibitors on renal function have been demonstrated in the EMPA‐REG OUTCOME study, four patients who developed acute coronary syndrome within 2 months were excluded. The DAPA‐CKD study 8 also demonstrated that the dapagliflozin group showed a significant advantage over the placebo group in terms of renal functional progression or death, which was defined as the primary composite endpoint. However, the population in the DAPA‐CKD study included patients with CKD with or without T2DM but excluded the patients with acute coronary syndromes. The patients in our EMBODY trial were randomly assigned to an empagliflozin (10 mg/day) group or a placebo group within 2 weeks of AMI onset and were administered a drug during the relatively acute phase of AMI, within 2–12 weeks of onset. Therefore, this sub‐analysis of the EMBODY trial is the first published study to investigate the reno‐protective effect of an SGLT2 inhibitor in patients with T2DM and AMI. The present study also showed the renal protective effect of empagliflozin, an SGLT2 inhibitor, in patients with T2DM and AMI, similar to previous, large, randomized, placebo‐controlled trials, suggesting that early administration of SGLT2 inhibitors after the onset of AMI is recommended for renal protection.
Mechanisms underlying the reno‐protective effects of sodium–glucose cotransporter 2 inhibitors
A previous paper reported that the urinary glucose excretion effect of SGLT2 inhibitors indirectly lowered serum uric acid levels by inhibiting glucose transporter (GLUT) 9, which acts on uric acid reabsorption in the proximal tubules of the kidney. 18 SGLT2 inhibitors increase the concentration of glucose in the proximal tubules and inhibit glucose reabsorption into the lumen, resulting in transfer of glucose from the proximal tubules to the vasculature by a glucose concentration gradient via GLUT9. Uric acid reabsorption is then inhibited via a potential‐dependent urate transporter 1 in the lumen. 18 A previous meta‐analysis concluded that uric acid‐lowering therapy may be effective in retarding the progression of CKD. 19 , 20 The results of this study showed that, regardless of baseline eGFR, the introduction of empagliflozin significantly reduced serum uric acid levels: upon stratified analysis of the baseline eGFR ≥ 60 group, empagliflozin demonstrated reno‐protective effects when compared with placebo; furthermore, there was a significant correlation between serum uric acid levels and renal functional markers, which are consistent with previous papers. 19 , 20
In addition to the earlier mechanism of lowering of serum uric acid levels, secondary factors such as suppression of the sympathetic nervous system and decreased BP and BW may contribute to the improvement of renal function. 21 The EMBODY trial and previous studies have shown that SGLT2 inhibitors have a sympathetic depressant effect, and reduced central sympathetic nervous system activation is thought to be an important mechanism for reducing renal SGLT2 expression. 22
The concept of tubulo‐glomerular feedback (TGF) is one of the mechanisms underlying the reno‐protective effects of SGLT2 inhibitors. 23 Increased SGLT2 induces increased proximal tubular reabsorption of not only glucose but also sodium. 24 This reduces sodium chloride and fluid delivery from the proximal tubule to the macula densa present downstream, thereby causing glomerular hyperfiltration via impaired TGF. Glomerular hyperfiltration increases sodium transport and oxygen consumption in the kidney, particularly in the proximal tubules, 25 consequently causing kidney damage. Therefore, diabetes‐induced glomerular hyperfiltration is a major risk factor for the subsequent development of diabetic kidney disease. 26 Furthermore, contraction of afferent arterioles and dilation of efferent arterioles via the TGF mechanism by SGLT2 inhibitors lead to correction of glomerular hypertension. 27 , 28 Our findings of significant renal protection in the eGFR ≥ 60 group and the correlation between serum uric acid level and eGFR are consistent with this mechanism. The reno‐protective effect of SGLT2 inhibitors has been shown to be more pronounced in the group of patients with relatively maintained GFR, that is, those with early nephropathy.
Trial limitations
This study had several limitations. First, this study included a small number of cases and a relatively short follow‐up period of 24 weeks. This may be why, regarding renal functional markers, there was a significant difference in the intra‐group comparison but not in the intergroup comparison. If the follow‐up period was further extended, the slopes and trends in the transition of renal function markers may have been different. Larger‐scale randomized controlled trials for patients with AMI are desired in the future. Second, we excluded patients treated with insulin, glucagon‐like peptide 1 analogues, or high doses of sulfonylureas and patients with HbA1c ≥ 10%. Third, optimal medical therapy, including use of renin–angiotensin system inhibitors, was at the discretion of the attending physician. However, there was no difference among research institutes. Fourth, the EMBODY trial was not designed with renal function as the primary endpoint; therefore, results of this sub‐analysis are not sufficient to confirm the renal protective effect of SGLT2 inhibitors, considering the sample size.
Conclusions
Our study findings suggested that early administration of empagliflozin to patients with T2DM and AMI, particularly those in whom the initial eGFR is relatively preserved at 60 mL/min/1.73 m2 or higher, suppresses the progression of renal dysfunction. The mechanism underlying the reno‐protective effect of SGLT2 inhibitors may involve a reduction of serum uric acid levels. Larger prospective studies are needed to confirm whether early introduction of SGLT2 in patients with T2DM and AMI improves long‐term renal outcomes and prognosis.
Conflict of interest
K.M., Y.K., Y.H., S.T., Y.T., K.Y., Y.I., T.Y., H.T., Y.T., K.A., M. Miyamoto, Y.M., E.K., M. Maruyama, M.O., and J.T. declare no conflicts of interest. W.S. has received honorariums and research grants from Boehringer Ingelheim.
Funding
This work was supported by Boehringer Ingelheim and Eli Lilly and Company (Grant Number 1245‐0175). The funding companies had no role in the design of the study; collection, analysis, and interpretation of data; or in the writing of the manuscript.
Supporting information
Table S1. Comparison of parameters between groups with eGFR 45–59.
Table S2. Comparison of parameters between groups with eGFR ≥ 60.
Table S3. Correlation between renal function markers and various parameters in the eGFR 45–59 group.
Table S4. Correlation between renal function markers and various parameters in the eGFR ≥ 60 group.
Acknowledgements
The authors wish to thank all the staff and patients who participated in the EMBODY trial. Current site investigators of the EMBODY trial include Department of Cardiovascular Medicine, Nippon Medical School, Tokyo, Japan: Reiko Shiomura, Isamu Fukuizumi, Junya Matsuda, Satsuki Noma, Jun Nakata, and Hideki Miyachi; Department of Cardiovascular Medicine, Nippon Medical School Chiba Hokuso Hospital, Chiba, Japan: Takahiro Todoroki, Takeshi Ikeda, Tomoyo Miyakuni, Masato Matsushita, Hirotake Okazaki, Akihiro Shirakabe, Nobuaki Kobayashi, Masamitsu Takano, and Yoshihiko Seino; Department of Cardiovascular Medicine, Nippon Medical School Tama Nagayama Hospital, Tokyo, Japan: Yugo Nishi, Keishi Suzuki, Junsuke Shibuya, Tsunenori Saito, and Hiroyuki Nakano; Department of Cardiovascular Medicine, Nippon Medical School Musashi Kosugi Hospital, Tokyo, Japan: Kenji Nakama, Ippei Tsuboi, and Yusuke Hosokawa; and Department of Cardiovascular Medicine, Shizuoka Medical Center, Shizuoka, Japan: Hidekazu Kawanaka and Michio Ogano. Consultancy and a part of trial management were provided by DOT WORLD Co., Ltd.
Mozawa, K. , Kubota, Y. , Hoshika, Y. , Tara, S. , Tokita, Y. , Yodogawa, K. , Iwasaki, Y. , Yamamoto, T. , Takano, H. , Tsukada, Y. , Asai, K. , Miyamoto, M. , Miyauchi, Y. , Kodani, E. , Maruyama, M. , Tanabe, J. , and Shimizu, W. (2021) Empagliflozin confers reno‐protection in acute myocardial infarction and type 2 diabetes mellitus. ESC Heart Failure, 8: 4161–4173. 10.1002/ehf2.13509.
References
- 1. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet 2010; 375: 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heise T, Seewaldt‐Becker E, Macha S, Hantel S, Pinnetti S, Seman L, Woerle HJ. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks' treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab 2013; 15: 613–621. [DOI] [PubMed] [Google Scholar]
- 3. Katayama S, Hatano M, Issiki M. Clinical features and therapeutic perspectives on hypertension in diabetics. Hypertens Res 2018; 41: 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 5. Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Barrett TD, Weidner‐Wells M, Deng H, Matthews DR, Neal B. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol 2018; 6: 691–704. [DOI] [PubMed] [Google Scholar]
- 6. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 7. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 8. Heerspink HJL, Stefánsson BV, Correa‐Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446. [DOI] [PubMed] [Google Scholar]
- 9. Shimizu W, Kubota Y, Hoshika Y, Mozawa K, Tara S, Tokita Y, Yodogawa K, Iwasaki YK, Yamamoto T, Takano H, Tsukada Y, Asai K, Miyamoto M, Miyauchi Y, Kodani E, Ishikawa M, Maruyama M, Ogano M, Tanabe J. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol 2020; 19: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kubota Y, Yamamoto T, Tara S, Tokita Y, Yodogawa K, Iwasaki Y, Takano H, Tsukada Y, Asai K, Miyamoto M, Miyauchi Y, Kodani E, Sato N, Tanabe J, Shimizu W. Effect of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: rationale. Diabetes Ther 2018; 9: 2107–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127: e362–e425. [DOI] [PubMed] [Google Scholar]
- 12. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130: 2354–2394. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67: 2089–2100. [DOI] [PubMed] [Google Scholar]
- 14. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Coresh J, Bolton K, Culleton B, Harvey KS, Ikizler TA, Cynda AJ, Kausz A, Kimmel PL, Kusek J, Levin A, Minaker KL, Nelson R, Rennke H, Steffes M, Witten B, Hogg RJ, Furth S, Lemley KV, Portman RJ. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–S266. [PubMed] [Google Scholar]
- 17. Kohagura K, Yamasaki H, Takano H, Ohya Y, Seino Y. Luseogliflozin, a sodium‐glucose cotransporter 2 inhibitor, preserves renal function irrespective of acute changes in the estimated glomerular filtration rate in Japanese patients with type 2 diabetes. Hypertens Res 2020; 43: 876–883. [DOI] [PubMed] [Google Scholar]
- 18. Chino Y, Samukawa Y, Sakai S, Nakai Y, Yamaguchi J, Nakanishi T, Tamai I. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos 2014; 35: 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu X, Zhai T, Ma R, Luo C, Wang H, Liu L. Effects of uric acid‐lowering therapy on the progression of chronic kidney disease: a systematic review and meta‐analysis. Ren Fail 2018; 40: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanji T, Gandhi M, Clase CM, Yang R. Urate lowering therapy to improve renal outcomes in patients with chronic kidney disease: systematic review and meta‐analysis. BMC Nephrol 2015; 16: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cherney DZI, Cooper ME, Tikkanen I, Pfarr E, Johansen OE, Woerle HJ, Broedl UC, Lund SS. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int 2018; 93: 231–244. [DOI] [PubMed] [Google Scholar]
- 22. Motoki H, Masuda I, Yasuno S, Oba K, Shoin W, Usami S, Saito Y, Waki M, Komatsu M, Ueshima K, Nakagawa Y, Son C, Yonemitsu S, Hiramitsu S, Konda M, Onishi K, Kuwahara K. Rationale and design of the EMPYREAN study. ESC Heart Fail 2020; 7: 3134–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cherney DZ, Perkins BA. Sodium‐glucose cotransporter 2 inhibition in type 1 diabetes: simultaneous glucose lowering and renal protection? Can J Diabetes 2014; 38: 356–363. [DOI] [PubMed] [Google Scholar]
- 24. Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 2017; 60: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, Espadero RM, Woerle HJ, Broedl UC, Johansen OE. SGLT‐2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015; 12: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Layton AT, Vallon V, Edwards A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am J Physiol Renal Physiol 2016; 310: F1269–F1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta‐analysis. Diabetologia 2009; 52: 691–697. [DOI] [PubMed] [Google Scholar]
- 28. van Bommel EJM, Muskiet MHA, van Baar MJB, Tonneijck L, Smits MM, Emanuel AL, Bozovic A, Danser AHJ, Geurts F, Hoorn EJ, Touw DJ, Larsen EL, Poulsen HE, Kramer MHH, Nieuwdorp M, Joles JA, van Raalte DH. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post‐glomerular vasodilatation rather than pre‐glomerular vasoconstriction in metformin‐treated patients with type 2 diabetes in the randomized, double‐blind RED trial. Kidney Int 2020; 97: 202–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of parameters between groups with eGFR 45–59.
Table S2. Comparison of parameters between groups with eGFR ≥ 60.
Table S3. Correlation between renal function markers and various parameters in the eGFR 45–59 group.
Table S4. Correlation between renal function markers and various parameters in the eGFR ≥ 60 group.


