Abstract
Aims
There is a lack of diagnostic and therapeutic options for patients with atrial cardiomyopathy and paroxysmal atrial fibrillation. Interestingly, an abnormal P‐wave terminal force in electrocardiogram lead V1 (PTFV1) has been associated with atrial cardiomyopathy, but this association is poorly understood. We investigated PTFV1 as a marker for functional, electrical, and structural atrial remodelling.
Methods and results
Fifty‐six patients with acute myocardial infarction and 13 kidney donors as control cohort prospectively underwent cardiac magnetic resonance imaging to evaluate the association between PTFV1 and functional remodelling (atrial strain). To further investigate underlying pathomechanisms, right atrial appendage biopsies were collected from 32 patients undergoing elective coronary artery bypass grafting. PTFV1 was assessed as the product of negative P‐wave amplitude and duration in lead V1 and defined as abnormal if ≥4000 ms*μV. Activity of cardiac Ca/calmodulin‐dependent protein kinase II (CaMKII) was determined by a specific HDAC4 pull‐down assay as a surrogate for electrical remodelling. Atrial fibrosis was quantified using Masson's trichrome staining as a measure for structural remodelling. Multivariate regression analyses were performed to account for potential confounders. A total of 16/56 (29%) of patients with acute myocardial infarction, 3/13 (23%) of kidney donors, and 15/32 (47%) of patients undergoing coronary artery bypass grafting showed an abnormal PTFV1. In patients with acute myocardial infarction, left atrial (LA) strain was significantly reduced in the subgroup with an abnormal PTFV1 (LA reservoir strain: 32.28 ± 12.86% vs. 22.75 ± 13.94%, P = 0.018; LA conduit strain: 18.87 ± 10.34% vs. 10.17 ± 8.26%, P = 0.004). Abnormal PTFV1 showed a negative correlation with LA conduit strain independent from clinical covariates (coefficient B: −7.336, 95% confidence interval −13.577 to −1.095, P = 0.022). CaMKII activity was significantly increased from (normalized to CaMKII expression) 0.87 ± 0.17 to 1.46 ± 0.15 in patients with an abnormal PTFV1 (P = 0.047). This increase in patients with an abnormal PTFV1 was independent from clinical covariates (coefficient B: 0.542, 95% confidence interval 0.057 to 1.027, P = 0.031). Atrial fibrosis was significantly lower with 12.32 ± 1.63% in patients with an abnormal PTFV1 (vs. 20.50 ± 2.09%, P = 0.006), suggesting PTFV1 to be a marker for electrical but not structural remodelling.
Conclusions
Abnormal PTFV1 is an independent predictor for impaired atrial function and for electrical but not for structural remodelling. PTFV1 may be a promising tool to evaluate patients for atrial cardiomyopathy and for risk of atrial fibrillation.
Keywords: Atrial cardiomyopathy, Atrial fibrillation, Atrial strain, CaMKII, Cardiac magnetic resonance imaging, PTFV1
Introduction
Atrial fibrillation (AF) is a widespread disease with an increasing prevalence and a great socio‐economic relevance, but treatment options and early diagnostic tools are still limited. 1 , 2 Atrial cardiomyopathy summarizes pathological functional, electrical, and structural remodelling in the atria. 2 In this context, atrial fibrosis as an unspecific reaction to many pathologic stimuli such as increased wall stress or inflammation, and atrial fibrosis constitutes a key finding in atrial cardiomyopathy. 2 Inflammation and reactive oxygen species production lead to activation of the interleukin‐6‐related MEK5‐ERK5 and STAT‐3 pathways, which are linked to fibrotic myocardial remodelling. 2 Arterial hypertension activates the renin–angiotensin–aldosterone axis, which then activates profibrotic pathways. 2 Fibrotic changes in the atrial wall structure are already found in early stages of lone paroxysmal AF, when atrial function and electrical conduction assessed with electrocardiogram (ECG) and echocardiography are still unchanged. Progressing fibrosis can lead to conduction abnormalities and is the structural correlate for re‐entry mechanisms. Furthermore, loss of viable myocytes and increased wall tissue stiffness due to excessive fibrosis eventually reduce atrial function. 2 Atrial fibrosis therefore is a hallmark of atrial cardiomyopathy. 2
Interestingly, atrial dysfunction measured volumetrically as increased size or reduced atrial ejection fraction has been associated with adverse cardiovascular outcome such as higher risk of stroke, 3 new onset of AF, 4 and higher mortality after myocardial infarction (MI). 2 , 5 Importantly, up to one‐fourth of all strokes is considered cryptogenic with no apparent cause. 6 Many of these strokes are cardioembolic, but paroxysmal AF may only be detected after extensive long‐term monitoring. 6 Therefore, early identification of atrial remodelling as a precursor of AF is of utmost importance to identify patients at risk for stroke. Unfortunately, current diagnostic tools are limited. In contrast to standard evaluation of atrial volumetry, analysis of myocardial deformation via strain analysis allows for more detailed assessment of atrial function. 7 Also, reduced strain correlates with atrial fibrofatty remodelling. 8 Despite these advantages, it is not yet routinely employed in clinical practice due to the limited availability of the necessary hardware and software. Interestingly, several novel ECG indices for atrial cardiomyopathy have been proposed. Among these, an abnormal P‐wave terminal force in lead V1 (PTFV1) has been shown to be an independent predictor for AF, stroke, and cardiac death or hospitalization for heart failure in patients with prior MI. 5 , 9 , 10 Abnormal PTFV1 is currently evaluated as a risk marker for secondary prevention of patients with cryptogenic stroke. 11 However, the underlying mechanisms for the development of abnormal PTFV1 and the consequences for atrial function are unknown. Interestingly, an increased Ca/calmodulin‐dependent protein kinase II (CaMKII)‐dependent dysregulation of cardiomyocytes ion homeostasis has already been associated with atrial pathologies. 1 , 2 , 12 , 13 , 14
The aim of the present study was to evaluate the link between abnormal PTFV1, atrial strain, and atrial CaMKII‐dependent pro‐arrhythmic activity in high‐risk patients with cardiovascular disease. This is of emerging interest because several CaMKII inhibitors are under preclinical consideration and have already been successfully tested in human myocardium. 13
Methods
An extended description of all materials and methods can be found in the Supporting Information. To avoid the possibility of unintentional distribution of private patient data, patient information can only be made available after written informed consent has been given by each patient for a specific request.
Study approval and design
For this study, patient data, cardiac magnetic resonance imaging (CMR), and specimens of two distinct studies and one control cohort were used. All studies were approved by the local ethics committee and are in accordance with the Declaration of Helsinki (first released in 1964, most recent revision 2013). Each patient has given his written informed consent prior to inclusion in this study.
Atrial CMR strain measurements were performed as a sub‐analysis of a prospective observational study in 56 patients with a first acute MI that was treated with percutaneous coronary intervention. 15 In order to report the range of physiological atrial strain, CMR acquisitions from 13 age‐matched and gender‐matched control individuals (potential living kidney donors) 16 were also analysed for atrial strain.
Atrial biopsies were collected from 32 patients who underwent elective coronary artery bypass grafting (CABG) in the prospective observational study CONSIDER‐AF. 17
Quantification of P‐wave terminal force in lead V1
P‐wave terminal force was defined by the negative area of the P‐wave in ECG lead V1 (PTFV1; Figure 1A ). This area was quantified by multiplying duration and amplitude (ms*μV). A PTFV1 of at least 4000 ms*μV (Figure 1A ) was defined as abnormal, which is in accordance with previous studies. 18
Figure 1.
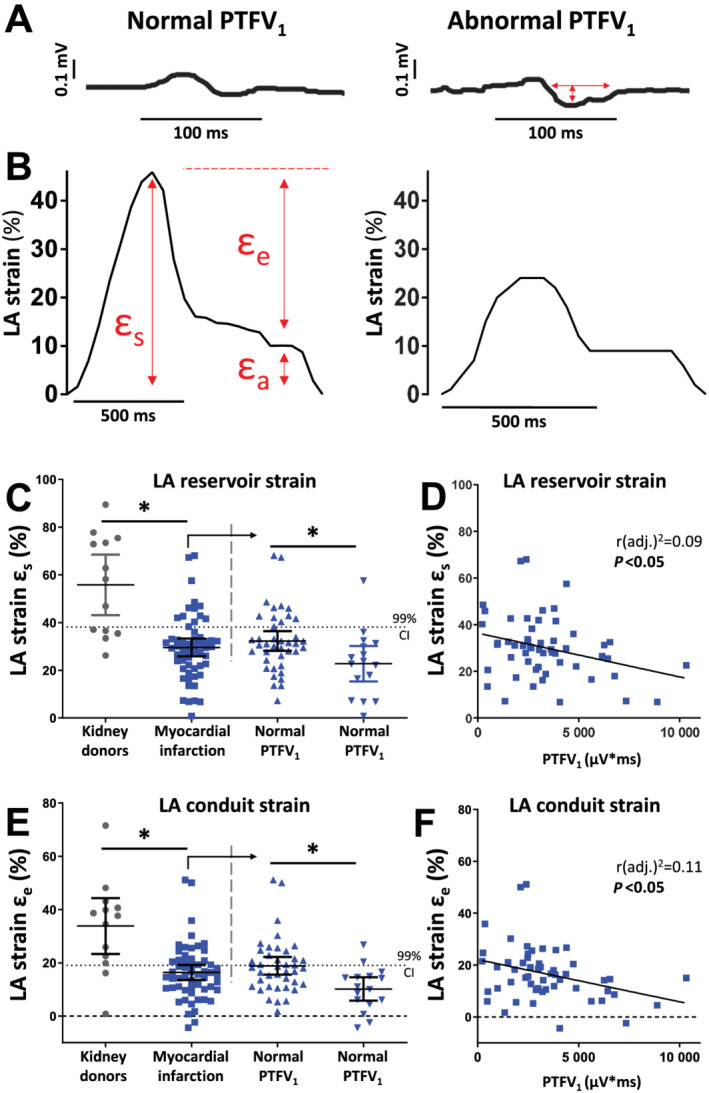
An abnormal PTFV1 indicates reduced atrial function. (A) Tracing of an ECG and LA strain in a patient with normal and abnormal PTFV1. Red arrows indicate measured values. PTFV1 is calculated as the product of the duration and depth of the negative deflection of biphasic P‐waves in the precordial ECG lead V1. (B) CMR feature tracking tracings of global longitudinal strain of the LA are used to calculate atrial strain parameters for reservoir (εs) and conduit (εe) function. (C–F) Left atrial reservoir and conduit function for the kidney donors (n = 13, grey dots) and the myocardial infarction group (n = 56, blue squares); the dotted line represents the lower 99% CI of the kidney donors; the myocardial infarction group is further divided in normal and abnormal PTFV1. (C) Left atrial reservoir function strain. (D) Correlation of PTFV1 and LA reservoir strain. (E) Left atrial conduit function strain. (F) Correlation of PTFV1 and LA conduit strain. CI, confidence interval; LA, left atrial; PTFV1, P‐wave terminal force V1; ε, strain. *P < 0.05, Student's t‐test. Exact values of means and P‐values are listed in Supporting Information, Table S2 .
Atrial function
To assess atrial function, CMR strain measurements were performed in patients with acute MI and in kidney donors. Longitudinal atrial strain was computed as (L1 − L0)/L0, where L0 is the resting/reference length [left ventricular (LV) end‐diastole] and L1 is the change of atrial myocardial length throughout the cardiac cycle. Atrial strain was analysed for total strain (εs, corresponding to atrial reservoir function), passive strain (εe, corresponding to atrial conduit function), and active strain (εa, corresponding to atrial booster function; Figure 1B ). Strain rate was calculated as change in ε over time: peak positive strain rate (corresponding to atrial reservoir function), peak early negative strain rate (corresponding to atrial conduit function), and peak late negative strain rate (corresponding to atrial booster function).
Atrial electrical remodelling
Electrical remodelling was analysed in right atrial appendage biopsies of patients undergoing elective CABG. CaMKII activity was measured by a specific assay [expressed in (normalized to CaMKII expression)] and was used as a surrogate for atrial electrical remodelling. Moreover, microdissected trabeculae were electrically field stimulated, and the severity of arrhythmias was classified. 12
Atrial structural remodelling
Volumetric atrial parameters were assessed by CMR in patients with acute MI and by echocardiography in patients undergoing CABG. The presence of fibrosis was histologically measured in atrial biopsies of the latter population.
Statistics
All investigators were blinded to the clinical data during the conduction and analysis of all experiments. Continuous clinical data are presented as means ± standard deviation, and categorical variables as total number with relative proportion. Experimental data are reported as means ± standard error of the mean, based on a single value for each patient. For the assessment of baseline distribution, Student's t‐test, Mann–Whitney test, and ANOVA for continuous variables and χ 2 or Fisher's exact test for discrete variables were used as appropriate. For univariate regression analysis of left atrial (LA) conduit strain and abnormal PTFV1, based on clinical and pathophysiological considerations, we chose the factors age, gender, body mass index, renal function [estimated glomerular filtration rate (eGFR)], LV function (ejection fraction), N‐terminal pro‐brain natriuretic peptide (NT‐pro‐BNP), MI with ST elevation, maximal levels for creatine kinase (CK max), and systolic blood pressure as important clinical covariates. Based on these univariate regression analyses, the factors with a P‐value equal to or less than 0.3 were included in a multivariate regression model [age, LV ejection fraction (LVEF), NT‐pro‐BNP, and eGFR]. For univariate regression analysis of CaMKII activity and PTFV1, based on clinical and pathophysiological considerations, we included age, gender, body mass index, existing paroxysmal AF, existing heart failure, and renal function (eGFR). Based on these univariate regression analyses, the factors with a P‐value equal to or less than 0.3 were included in a multivariate regression model (only PTFV1 and age). We used IBM SPSS Statistics 25 for univariate or multivariate linear regression analyses. Statistical significance was defined by two‐sided P‐values below 0.05.
Results
Characteristics of patients with cardiac magnetic resonance imaging analyses
The baseline characteristics of kidney donors (n = 13) and patients after acute MI (n = 56) are listed in Supporting Information, Table S1 . A total of 3/13 (23%) of the kidney donors had a PTFV1 ≥ 4000 ms*μV. Table 1 presents the baseline characteristics of the MI patient cohort stratified into normal (n = 40) and abnormal PTFV1 (n = 16). Patients with abnormal PTFV1 were more likely to present with reduced LVEF and elevated NT‐pro‐BNP levels at discharge (Table 1 ).
Table 1.
Baseline characteristics of patients with acute myocardial infarction
| Normal PTFV1 (n = 40) | Abnormal PTFV1 (n = 16) | P‐value | |
|---|---|---|---|
| PTFV1 (ms*μV), mean ± SD | 2353.5 ± 1072.2 | 6060.8 ± 1808.6 | |
| Age (years), mean ± SD | 53.9 ± 9.9 | 57.9 ± 9.6 | 0.173T |
| Male, n (%) | 34 (85.0%) | 11 (68.7%) | 0.263F |
| Body mass index (kg/m2), mean ± SD | 28.5 ± 3.1 | 28.8 ± 4.0 | 0.771T |
| Cardiovascular risk factors | |||
| Arterial hypertension, n (%) | 19 (47.5%) | 9 (60%) | 0.408Chi |
| Diabetes mellitus, n (%) | 6 (15%) | 3 (20%) | 0.705F |
| Current or previous smoker, n (%) | 30 (75%) | 11 (73.5%) | 0.741F |
| Hypercholesterolaemia, n (%) | 12 (30%) | 5 (33.3%) | 1.000F |
| Heart and renal function | |||
| STEMI, n (%) | 3 (8.8%) | 4 (33.3%) | 0.094F |
| CK max (U/L), mean ± SD | 1993.5 ± 1393.2 | 2232.1 ± 1588.6 | 0.59T |
| NT‐pro‐BNP at discharge, (pg/mL), mean ± SD | 774.5 ± 835.6 | 2201.2 ± 1390.4 | <0.001 T |
| LVEF (%), mean ± SD | 48.7 ± 7.5 | 42.7 ± 11.8 | 0.037 T |
| LVEF classification | 0.102F | ||
| Normal EF ≥ 50%, n (%) | 22 (55.0%) | 6 (37.5%) | |
| Mildly reduced EF 40–49%, n (%) | 15 (37.5%) | 5 (31.3%) | |
| Moderately reduced EF 30–39%, n (%) | 3 (7.5%) | 4 (25.0%) | |
| Severely reduced EF ≤ 30%, n (%) | 0 (0%) | 1 (6.3%) | |
| LV mass index (g/m2), mean ± SD | 70.0 ± 12.6 | 80.8 ± 14.7 | 0.006 T |
| LV end‐diastolic volume (mL), mean ± SD | 155.8 ± 38.3 | 170.8 ± 38.1 | 0.161T |
| LAVI (mL/m2), mean ± SD | 27.1 ± 12.8 | 33.2 ± 17.1 | 0.208T |
| RV ejection fraction (%), mean ± SD | 59.6 ± 8.2 | 57.7 ± 10.5 | 0.500T |
| RV end‐diastolic volume (mL), mean ± SD | 134.4 ± 29.5 | 128.9 ± 28.5 | 0.525T |
| RA volume index (mL/m2), mean ± SD | 13.5 ± 2.3 | 12.7 ± 2.4 | 0.287T |
| Systolic blood pressure (mmHg), mean ± SD | 127.4 ± 22.8 | 127.7 ± 17.7 | 0.970T |
| eGFR (mL/min/1.73 m2), mean ± SD | 95.2 ± 16.5 | 83.6 ± 28.0 | 0.065T |
| Medication | |||
| ACE‐inhibitor/AT1‐blocker, n (%) | 39 (97.5%) | 16 (100%) | 1.000F |
| Beta‐blocker, n (%) | 39 (97.5%) | 15 (93.7%) | 0.494F |
| Aldosterone receptor antagonists, n (%) | 18 (45%) | 11 (68.7%) | 0.144F |
| Loop diuretics, n (%) | 16 (40%) | 9 (56.2%) | 0.269Chi |
ACE, angiotensin‐converting enzyme; BNP, brain natriuretic peptide; Chi, χ 2 test; CK, creatine kinase; EF, ejection fraction; eGFR, estimated glomerular filtration rate; F, Fisher's exact test; LA, left atrial; LV, left ventricular; NT‐pro‐BNP, N‐terminal pro‐brain natriuretic peptide; PTFV1, P‐wave terminal force in lead V1; RA, right atrial; RV, right ventricular; SD, standard deviation; STEMI, ST‐elevation myocardial infarction; T, Student's t‐test.
Clinical characteristics of patients with acute myocardial infarction and cardiac magnetic resonance imaging cine data. Bold values mean statistical significance calculated by two‐sided Student's t‐test, χ 2 test, or Fisher's exact test, as appropriate.
Left atrial function is impaired in patients with abnormal P‐wave terminal force in lead V1
As there exist only sparse and incongruent data on normal atrial strain values, we measured LA strain in kidney donors. In accordance with impaired LA contractile function, LA reservoir, conduit, and booster function were all significantly depressed in patients after MI compared with kidney donors (Figure 1C–F , Supporting Information, Figure S3 , and Supporting Information, Table S2 ).
Importantly, within the cohort presenting with MI, patients with an abnormal PTFV1 showed significantly more impaired LA reservoir and conduit strain compared with patients with normal PTFV1 (Figure 1C + E ). In accordance, the magnitude of PTFV1 and strain for LA reservoir and LA conduit function correlated significantly negative (Figure 1D + F ). Strain rate is calculated as the change in strain over time, which means that reservoir strain rate has positive values whereas conduit and booster strain rates have negative values. Similarly, in patients with abnormal PTFV1, LA reservoir strain rate was significantly lower, but LA conduit strain rate was significantly larger compared with MI patient with normal PTFV1 (Supporting Information, Figure S3 ). There was also a significant correlation between the magnitude PTFV1 and both the latter parameters (Supporting Information, Figure S3 ).
In contrast, there were no differences between groups for LA booster function, which describes the active atrial contraction (Supporting Information, Table S2 ).
Diagnostic value of P‐wave terminal force in lead V1 for reduced atrial function
Baseline characteristics (Table 1 ) suggested that patients with heart failure were more likely to show abnormal PTFV1 and ventricular contractile dysfunction that can substantially impact atrial strain. Therefore, we have performed univariate and multivariate linear regression for reduced LA conduit function and PTFV1 and important clinical confounders (Table 2 ). We have chosen LA εe among the strain parameters, because it showed the strongest relationship with PTFV1 (largest r 2; Figure 1F and Supporting Information, Figure S3C ).
Table 2.
Abnormal PTFV1 predicts independently pathological atrial function
| n = 56 | Univariate linear regression analysis |
Multivariate linear regression analysis Adj. r 2 = 0.402; P < 0.001 |
||
|---|---|---|---|---|
| LA conduit strain (%) | B (95% CI) | P‐value | B (95% CI) | P‐value |
| Abnormal PTFV1 (≥4000 μV*ms) | −8.703 (−14.521; −2.885) | 0.004 | −7.336 (−13.577; −1.095) | 0.022 |
| Age (years) | −0.426 (−0.691; −0.160) | 0.002 | −0.416 (−0.683; −0.149) | 0.003 |
| LVEF (%) | 0.548 (0.280; 0.815) | <0.001 | 0.419 (1.121; 0.717) | 0.007 |
| NT‐pro‐BNP (pg/mL) | −0.002 (−0.004; 0.000) | 0.096 | 0.002 (−0.001; 0.004) | 0.171 |
| CK max (U/L) | −0.002 (−0.004; 0.000) | 0.121 | −0.001 (−0.003; 0.001) | 0.478 |
| STEMI | −1.158 (−13.077; 10.762) | 0.845 | ||
| Male | −0.893 (−8.034; 6.248) | 0.803 | ||
| Body mass index (kg/m2) | −0.186 (−1.059; 0.687) | 0.671 | ||
| systolic blood pressure (mmHg) | 0.025 (−0.111; 0.160) | 0.717 | ||
CI, confidence interval; CK, creatine kinase; LA, left atrial; LVEF, left ventricular ejection fraction; NT‐pro‐BNP, N‐terminal pro‐brain natriuretic peptide; PTFV1, P‐wave terminal force in lead V1; STEMI, ST‐elevation myocardial infarction.
Linear regression analyses between an abnormal PTFV1 and left atrial conduit strain in patients with acute myocardial infarction. Bold font is used to highlight statistically significant P‐values < 0.05.
Univariate regression analysis revealed that besides PTFV1, the parameters age and LVEF significantly correlated with LA εe. Interestingly, incorporating age, eGFR, LVEF, and NT‐pro‐BNP in a multivariate model, abnormal PTFV1 was independently associated with impaired atrial strain, suggesting that PTFV1 might be a valuable independent marker for atrial function.
To investigate which clinical parameters influence the magnitude of PTFV1, we performed univariate regression analyses using parameters for cardiac function, cardiac injury, biometric data, and co‐morbidities (Supporting Information, Table S3 ). Besides LA strain, there was a significant association with NT‐pro‐BNP levels at discharge, and a strong trend for magnitude of LVEF confirming the importance of LV function for atrial electrical remodelling and corroborating the consideration of LV dysfunction as an important confounder.
Baseline characteristics of patients undergoing elective coronary artery bypass grafting
To investigate possible pathomechanisms for the development of a PTFV1‐detected atrial cardiomyopathy, we have analysed atrial biopsies of 32 patients undergoing elective CABG, stratified by a normal (n = 17) and an abnormal PTFV1 (n = 15, Supporting Information, Figure S2 and Supporting Information, Table S4 ). There were no differences between both groups regarding important clinical characteristics.
Abnormal P‐wave terminal force in lead V1 predicts atrial electrical remodelling
Because CaMKII is a key regulator of cardiac ion homeostasis and may underlie atrial electrical remodelling, 14 we measured CaMKII activity using a highly specific HDAC4 pull‐down assay. Intriguingly, CaMKII activity was significantly increased in patients with an abnormal PTFV1 (0.87 ± 0.17 vs. 1.46 ± 0.15, P = 0.047; Figure 2B ). There was also a significant positive correlation of CaMKII activity and the magnitude of PTFV1 (r 2 = 0.21, n = 20, P = 0.04; Figure 2C ).
Figure 2.
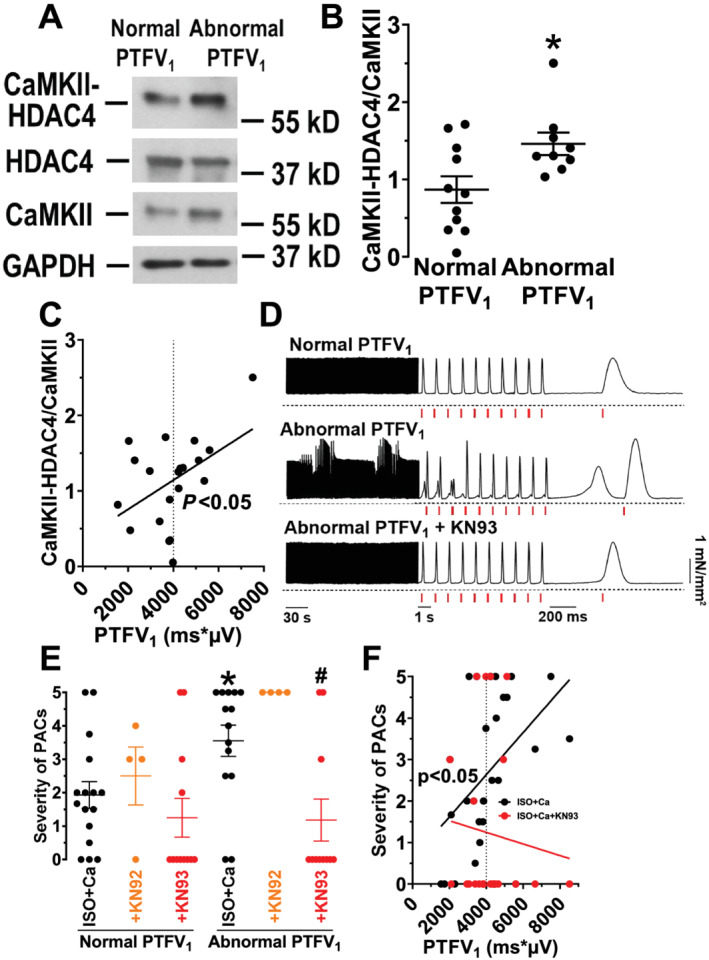
Electrical remodelling in patients with an abnormal PTFV1. (A) Original Western blot for the analysis of CaMKII activity, assessed by a specific HDAC4 (Histone Deacetylase 4) pull‐down assay in atrial homogenates of CABG patients with a normal and an abnormal PTFV1. (B) Densiometric analysis revealed a significantly increased CaMKII activity (CaMKII‐HDAC4 binding normalized to CaMKII expression) in patients with an abnormal PTFV1 (n = 9 vs. 11). (C) Moreover, linear regression analysis showed a significant positive correlation between PTFV1 and CaMKII activity (n = 20). (D) Representative original recordings of electrically stimulated trabeculae (stimulated contractions indicated by red vertical lines) for a patient with a normal PTFV1 and a patient with an abnormal PTFV1 before and after wash‐in of the CaMKII inhibitor KN93. (E) Intriguingly, the mean severity of premature atrial contractions (PACs) was significantly increased in patients with an abnormal PTFV1 (n = 14 vs. 16) but could be significantly reduced by CaMKII inhibition with KN93 (n = 11). Moreover, the inactive analogue KN92 did not show any anti‐arrhythmic effect. (F) Additionally, we observed a significant positive correlation between PTFV1 and the severity of PACs (n = 30). Interestingly, this correlation was completely abolished upon CaMKII inhibition with KN93 (n = 23). *P < 0.05 vs. normal PTFV1, # P < 0.05 vs. ISO + Ca, Mann–Whitney test, two‐way ANOVA post hoc corrected by Holm–Sidak, and linear regression analysis, as appropriate.
Because CaMKII has been shown to foster the occurrence of pro‐arrhythmic events, 13 , 14 we measured premature atrial contractions (PACs) in human atrial trabeculae, which are a surrogate for multicellular arrhythmias. 12 Interestingly, trabeculae from patients with an abnormal PTFV1 exhibited a significantly increased severity of PACs resulting in a significant positive correlation between the magnitude of PTFV1 and PACs severity (r 2 = 0.19, n = 30, P = 0.02; Figure 2D–F ). Intriguingly, specific CaMKII inhibition with KN93 significantly reduced the incidence and severity of atrial pro‐arrhythmic activity in patients with abnormal PTFV1. PAC severity decreased from 3.55 ± 0.47 (n = 14) to 1.18 ± 0.63 (n = 11, P = 0.008), a level that was comparable with patients with a normal PTFV1 (1.25 ± 0.58, n = 12; Figure 2E ). CaMKII inhibition with KN93 also abolished the positive correlation between magnitude of PTFV1 and PAC severity (Figure 2F ). In contrast, the inactive analogue KN92, which does not inhibit CaMKII, did not reduce the severity of PACs, suggesting that atrial pro‐arrhythmic activity in patients with an abnormal PTFV1 was due to CaMKII‐dependent signalling (Figure 2E ).
As presented in Supporting Information, Table S4 , the investigated patients had multiple co‐morbidities that could substantially confound our observations. To control for this potential limitation, we conducted univariate and multivariate linear regression analyses, accounting for age, sex, body mass index, existing paroxysmal AF, existing heart failure, and glomerular filtration rate. Most importantly, the correlations of abnormal PTFV1 with CaMKII activity (Table 3 ) or the severity of trabecular arrhythmias (Supporting Information, Table S5 ) were independent from potential confounders, suggesting that the magnitude of PTFV1 may be a good clinical measure for abnormal atrial CaMKII signalling.
Table 3.
Abnormal PTFV1 is an independent predictor for CaMKII activity
|
n = 20
|
Univariate linear regression analysis |
Multivariate linear regression analysis Adj. r 2 = 0.255; P = 0.032 |
||
|---|---|---|---|---|
| CaMKII activity (normalized to CaMKII expression) | B (95% CI) | P‐value | B (95% CI) | P‐value |
| Abnormal PTFV1 (≥4000 ms*μV) | 0.591 (0.106; 1.077) | 0.020 | 0.542 (0.057; 1.027) | 0.031 |
| Age (years) | −0.022 (−0.053; 0.008) | 0.143 | −0.017 (−0.045; 0.011) | 0.210 |
| Male | 0.032 (−0.620; 0.683) | 0.920 | ||
| Body mass index (kg/m2) | 0.012 (−0.058; 0.082) | 0.729 | ||
| Paroxysmal atrial fibrillation | 0.205 (−0.579; 0.988) | 0.590 | ||
| Existing heart failure | 0.035 (−0.540; 0.611) | 0.899 | ||
| eGFR (mL/min/1.73 m2) | −0.001 (−0.012; 0.009) | 0.781 | ||
CaMKII, Ca/calmodulin‐dependent protein kinase II; CI, confidence interval; eGFR, estimated glomerular filtration rate; PTFV1, P‐wave terminal force in lead V1.
Linear regression analyses between an abnormal PTFV1 and CaMKII activity in right atrial appendage biopsies of patients undergoing coronary artery bypass grafting. Bold font is used to highlight statistically significant P‐values < 0.05.
Abnormal P‐wave terminal force in lead V1 is not indicative of structural remodelling
In contrast to parameters of atrial function and electrical remodelling, CMR parameters of structural remodelling (e.g. LA volume index and LA fractional area change) showed no significant correlation with PTFV1 in patients after acute MI (Supporting Information, Table S3 ).
Consistent with this, echocardiographic measurements in patients undergoing elective CABG showed that an abnormal PTFV1 was not associated with atrial size (Supporting Information, Table S4 ). We further analysed structural remodelling by histological measurement of fibrosis in atrial biopsies of patients undergoing CABG. Surprisingly, the area of fibrotic tissue was significantly reduced in patients with an abnormal PTFV1 (12.32 ± 1.63%, n = 13 vs. 20.50 ± 2.09%, n = 17; Figure 3B ). In addition, there was a significant negative correlation between the magnitude of PTFV1 and the severity of atrial fibrosis (r 2 = 0.17, n = 30, P = 0.02; Figure 3B ). After accounting for potential clinical confounders, multivariable regression analysis revealed a significant and independent inverse association between abnormal PTFV1 and severity of atrial fibrosis (Supporting Information, Table S6 ). Accordingly, we found a significant negative correlation between the area of fibrotic tissue and CaMKII activity (r 2 = 0.36, n = 19, P = 0.006; Figure 3C ). Interestingly, only the amplitude, but not the duration of the terminal force, correlated significantly negative with the area of fibrotic tissue (r 2 = 0.21, n = 30, P = 0.01; Supporting Information, Figure S4 ).
Figure 3.
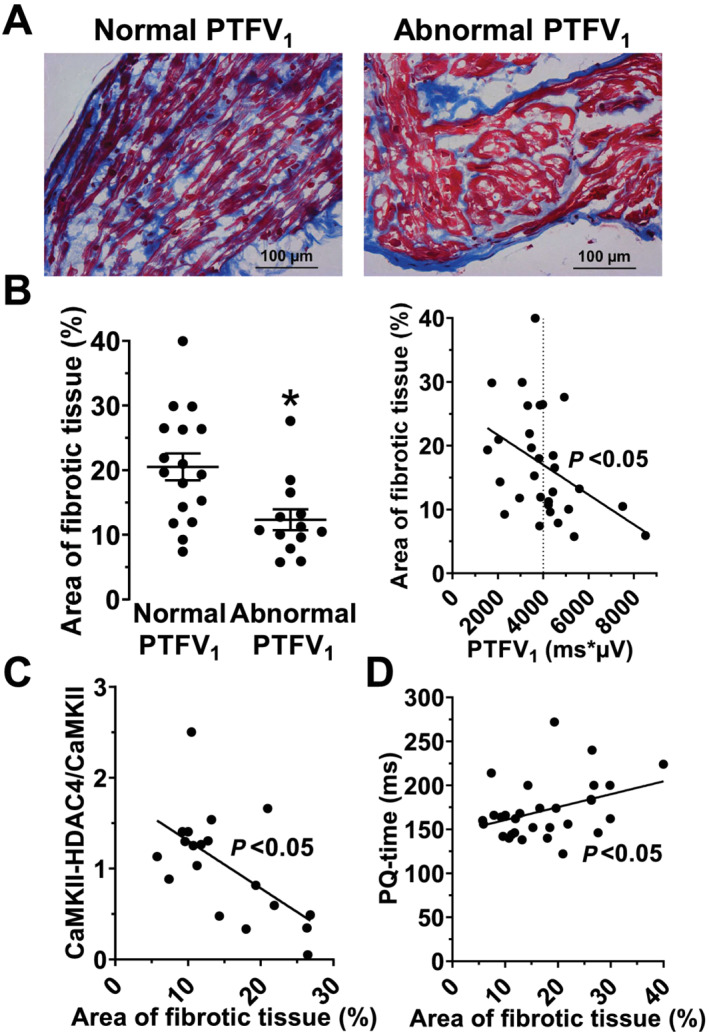
Atrial fibrosis is decreased in patients with an abnormal PTFV1. (A) Original micrographs showing myocardial fibrosis (Masson's trichrome staining) in trabeculae of CABG patients with a normal and an abnormal PTFV1 (400× magnification). (B) Interestingly, the area of myocardial fibrosis (%) was significantly reduced in patients with an abnormal PTFV1 (n = 13 vs. 17), leading to a significant negative correlation with the PTFV1 (n = 30). (C) Moreover, we also found the area of fibrosis correlating significantly negative with the CaMKII activity, indicating that active CaMKII can only be found in cardiomyocytes and not in fibrotic tissue (n = 19). (D) Additionally, we found the area of fibrosis correlating significantly positive with a slowed atrial conduction velocity (i.e. PQ time, n = 30). *P < 0.05, Mann–Whitney test and linear regression analysis, as appropriate.
In contrast to the amplitude, the duration of intra‐atrial and atrioventricular conduction appears to depend on the magnitude of fibrotic tissue. Interestingly, the PQ interval as a measure of atrial conduction correlated significantly positive with the area of fibrotic tissue (r 2 = 0.14, n = 30, P = 0.04; Figure 3D ).
Discussion
Atrial functional, electrical, and structural abnormalities such as reduced focal contractility, intermittent non‐recordable arrhythmias, or fibrosis have been suggested to result in a pro‐thrombotic and pro‐arrhythmogenic atrial disease called atrial cardiomyopathy. 2 , 6 However, urgently needed clinical markers that identify patients with atrial cardiomyopathy that are at increased risk for AF or stroke are currently missing. We show here that the presence of an abnormal PTFV1 significantly correlates with reduced atrial strain and increased CaMKII‐dependent atrial pro‐arrhythmic activity in contrast to structural atrial remodelling and independent from important clinical confounders (graphical abstract).
P‐wave terminal force in lead V1 is a marker for reduced atrial function
A large body of evidence has linked impaired atrial strain to reduced cardiovascular outcome. 4 , 19 Several studies have shown good feasibility and reproducibility of feature tracking assessment of LA strain parameters in healthy and in acutely ill patients. 20 , 21 , 22 Strain assessment of the LA in echocardiography and CMR is fairly well established but not yet routinely applied. 23 Reduced atrial strain is a sensitive marker of atrial dysfunction, and its evaluation improves the accuracy of risk stratification in multiple cardiac pathologies. LA strain measurement provides risk assessment for stroke in patients with AF in addition to the CHA2DS2‐VASc score. 24
Accordingly, our data show that LA strain is reduced after acute MI compared with kidney donor subjects (Figure 1 and Supporting Information, Figure S3 ). As there are sparse and incongruent data on normal LA strain values, 23 we included the strain data of the kidney donors to define pathological strain values as values below the lower 99% confidence interval (Figure 1C + E and Supporting Information, Figure S3B + C ). However, despite the useful additional information, CMR is not generally available in most settings of cardiovascular care.
In that respect, the presence of an abnormal PTFV1 derived from ubiquitously available ECG analysis has recently been associated with atrial remodelling. 2 , 6 Moreover, an abnormal PTFV1 predicts an increased risk for AF and was independently associated with the incidence for cryptogenic or cardioembolic stroke. 6 , 9 , 10 Additionally, an abnormal PTFV1 has been shown to be an independent predictor for hospitalization and for increased mortality due to cardiovascular disease. 5
However, the mechanisms that result in abnormal PTFV1 are poorly understood. It was hypothesized that altered haemodynamics may cause subtle deformation of the LA, which then changes the electrical vector in the ECG and elevates PTFV1 amplitude. 25
We show here that an abnormal PTFV1 was independently associated with impaired LA reservoir and conduit function (Figure 1 ). It appears reasonable to assume that PTFV1 may be influenced by a multitude of factors that alter not only atrial function but also ventricular function and haemodynamics. Importantly, we show here by linear regression that impaired LA conduit strain (besides NT‐pro‐BNP) was the strongest predictor for abnormal PTFV1, but LVEF despite showing a clear trend did not reach statistical significance (Supporting Information, Table S3 ).
Electrical remodelling and P‐wave terminal force in lead V1
Because atrial contractile function and atrial arrhythmogenesis have both been shown to be regulated by CaMKII, and increased expression and activity of CaMKII are hallmarks of contractile dysfunction and arrhythmias in patients with AF 26 and heart failure, 14 we measured atrial CaMKII activity in a high‐risk cohort of cardiovascular patients undergoing CABG. We show here that increased atrial CaMKII activity was significantly correlated with abnormal PTFV1 (Figure 2A–C ). Many previous studies investigating human myocardium were limited regarding scarce data about inclusion criteria, and patient characteristics with important co‐morbidities were often not available. The information about co‐morbidities, for example, heart failure or AF, however, are of utmost importance as these are tightly linked with atrial cardiomyopathy. Both heart failure and AF have already been shown to be associated with an increased CaMKII‐dependent pro‐arrhythmic activity. 12 , 13 , 14 To account for this, we conducted multivariate linear regression analyses using the important clinical covariates age, gender, body mass index, existing paroxysmal AF, existing heart failure, and glomerular filtration rate. We show here that the increase of CaMKII activity in patients with an abnormal PTFV1 was independent from important co‐morbidities (Table 3 ).
Interestingly, it has been shown previously that increased CaMKII activity results in an impairment of cardiomyocyte Na and Ca homeostasis leading to increased cardiomyocyte triggered activity due to the promotion of early and delayed afterdepolarizations. 2 , 12 , 13 , 14
To better understand the relationship between an abnormal PTFV1, enhanced CaMKII signalling, and atrial arrhythmias, we measured atrial pro‐arrhythmic activity. We here show that the magnitude of PTFV1 correlated significantly and independently positive with the severity of trabecular arrhythmias. Moreover, selective CaMKII inhibition with KN93 significantly reduced the severity of trabecular arrhythmias, indicating the pivotal role of CaMKII for arrhythmogenesis in patients with an abnormal PTFV1 (Figure 2E ).
An abnormal P‐wave terminal force in lead V1 does not indicate structural remodelling
Besides disturbed cardiomyocyte Na and Ca homeostasis, structural remodelling (e.g. fibrosis) is another important feature of atrial cardiomyopathy. 2 In the present study, linear regression models revealed no significant correlation between PTFV1 and the volumetric LA parameters area, fractional area change, and volume index (Supporting Information, Table S3 for CMR data and Supporting Information, Table S4 for echocardiographic data), suggesting that PTFV1 does not indicate LA enlargement. This is in accordance with a study in healthy athletes also showing no significant differences for median LA diameter and median LA end‐systolic volume in individuals with an abnormal PTFV1. 27
Interestingly, atrial structural remodelling in patients with atrial cardiomyopathy is frequently accompanied by increased fibrosis development. Moreover, long‐term tachycardia or AF itself have been shown to induce atrial fibrosis that is essential for the progression to permanent AF. 2 Therefore, we investigated the magnitude of fibrosis in atrial biopsies of high‐risk cardiovascular patients undergoing CABG. Surprisingly, we observed that patients with abnormal PTFV1 showed significantly less atrial fibrosis and that this decrease was independent from important clinical covariates (Figure 3 and Supporting Information, Table S6 ). At first glance, this result may appear counterintuitive, especially, because a previous study suggested that ventricular fibrosis may be increased in patients with abnormal PTFV1. 28 There are several explanations for this discrepancy. We have confirmed atrial fibrosis by histochemical analysis using Masson's trichrome stain. Tiffany Win and colleagues have used surrogate markers measured by magnetic resonance imaging (late gadolinium enhancement) and only for ventricular myocardium, which may be prone to misinterpretation. 28 Another explanation is based on the fact that only vital cardiomyocytes, but not fibrotic tissue, are electrically active. Thus, increased fibrosis—that is accompanied by a reduced number of vital cardiomyocytes—may be less able to generate terminal force amplitude. In our analysis, the magnitude of fibrosis was associated with reduced terminal force amplitude, which supports this hypothesis (Supporting Information, Figure S4 ). One could argue that, on the other hand, increased fibrosis may have slowed atrial conduction resulting in increased terminal force duration. We show here, however, that terminal force duration was not correlated with atrial fibrosis (Supporting Information, Figure S4 ), excluding the possibility that slowed atrial conduction contributed to the magnitude of PTFV1. In accordance, the magnitude of fibrosis was also negatively correlated to CaMKII activity (Figure 3C ), and the latter is almost exclusively expressed in cardiomyocytes and not in fibrotic tissue. This suggests that the magnitude of PTFV1 may be a good marker for increased electrical remodelling in vital cardiomyocytes possibly at earlier stages of atrial cardiomyopathy when structural remodelling is not yet prevailing. Interestingly, consistent with the concept of fibrosis slowing conduction, we observed a significant positive correlation between the duration of the PQ interval and the magnitude of fibrosis (Figure 3D ). A possible explanation for the different effect of fibrosis on intra‐atrial vs. atrioventricular conduction may be the very high atrial conduction reserve. 29 The conduction velocity in the AV node, on the other hand, may be much more sensitive to fibrosis.
Limitations
A potential limitation is that LA functional, electrical, and structural remodelling were measured in two distinct populations, which might confound our observations. Unfortunately, as standardized CMR acquisition and human atrial biopsies are each rare and difficult to obtain, there were no patients with both. At least, observations for PTFV1 and structural remodelling (volumetric CMR and echocardiographic measurements) were consistent in both populations.
Another potential limitation may be that the diagnosis of heart failure, which is frequently associated to CaMKII‐dependent pro‐arrhythmic activity and remodelling, was no prespecified inclusion criterium. However, substantial fractions of heart failure patients were present in the MI and CABG cohorts, and multivariate regression analyses revealed that LV dysfunction did not affect atrial strain or atrial structural remodelling in our cohorts. Nevertheless, we cannot exclude that in a more selected heart failure cohort, LV dysfunction would also result in impaired atrial strain and structural remodelling.
We used kidney donors as a control group who underwent a thorough medical evaluation to be eligible for kidney donation. Strain values of these presumed healthy individuals may therefore provide insight into normal strain ranges. However, the small number of 13 patients in this group may not allow broad generalizability of these results.
P‐wave terminal force in lead V1 may be a novel clinical marker for early atrial cardiomyopathy
Our data suggest PTFV1 to be an independent marker for early atrial cardiomyopathy, characterized by functional and electrical, but not yet irreversible structural remodelling, which may have several diagnostic and therapeutic implications. Firstly, we found PTFV1 predicting atrial pro‐arrhythmic activity. Paroxysmal AF may only be detected by very long‐term ECG monitoring and is often clinically inapparent, yet possibly resulting in severe sequelae like cardioembolic stroke. 1 , 6 Atrial cardiomyopathy may result in a pro‐thrombotic environment in absence of AF. 2 , 6 Therefore, PTFV1 may be a selection criterion for extended long‐term ECG monitoring.
Moreover, clinical evidence suggests that the extent of atrial fibrosis is a critical determinant for a successful atrial ablation procedure. 1 However, measurement of atrial fibrosis by CMR is not an easy task and far away from clinical routine. We found PTFV1 as a biomarker for reduced atrial fibrosis, which may help to identify the patients with the highest success rate for atrial ablation procedures.
Finally, an abnormal PTFV1 may even serve as a novel and readily available clinical marker for enhanced CaMKII‐dependent pro‐arrhythmic activity. This is of special relevance because several CaMKII inhibitors are currently under preclinical consideration. 13 Thus, an abnormal PTFV1 may guide future application of CaMKII inhibitors for the treatment of atrial cardiomyopathy.
Conflict of interest
M.A. received grants and personal fees from Philips Respironics (Murrysville, PA 15668), grants and personal fees from ResMed Germany (Martinsried, Germany), grants from the ResMed Foundation (La Jolla, CA 92037), personal fees from Boehringer‐Ingelheim, personal fees from Novartis, personal fees from Bresotec, and personal fees from NRI, outside the submitted work.
Funding
S.L. was supported by the ReForM A program of the Medical Faculty at the University of Regensburg. M.W. is funded by the ReForM B program of the Medical Faculty at the University of Regensburg. M.T. was supported by a research grant from the Else‐Kroener Fresenius Foundation (Else Kröner‐Fresenius‐Stiftung; 2020_EKEA.25). C.F. was supported by the ReForM A program of the Medical Faculty at the University of Regensburg and received a grant from the Deutsche Herzstiftung (German Heart Foundation) (F/15/20). L.S.M. is funded by Deutsche Forschungsgemeinschaft (DFG) grants MA 1982/5‐1 and MA 1982/7‐1. M.A. received grant support from the Else‐Kroener Fresenius Foundation (2018_A159). S.W. is funded by DFG grants WA 2539/4‐1, WA 2539/5‐1, WA 2539/7‐1, and WA 2539/8‐1. S.W. and L.S.M. are also supported by the DFG SFB 1350 grant (Project Number 387509280, TPA6) and are funded by the ReForM C program of the Medical Faculty at the University of Regensburg. CONSIDER‐AF was supported by grants from Philips Respironics (Murrysville, PA 15668) and the Medical Faculty at the University of Regensburg.
Open Access funding enabled and organized by Projekt DEAL.
Supporting information
Figure S1. Study flowchart for patients with acute MI.
Figure S2. Study flowchart for patients undergoing elective CABG.
Figure S3. Strain rate values for kidney donors and patients with acute MI.
Figure S4. Trabecular fibrosis alters electrical conduction.
Table S1: Baseline characteristics of all patients with CMR data.
Table S2: Atrial strain from CMR.
Table S3: Univariate linear regression for the magnitude of PTFV1.
Table S4: Baseline characteristics of patients undergoing CABG.
Table S5: Abnormal PTFV1 as an independent predictor for the severity of trabecular arrhythmias.
Table S6: Abnormal PTFV1 is negatively and independently associated with atrial fibrosis.
Acknowledgements
We greatly appreciate the excellent technical assistance of Andrea Ochsenkühn, Gabriela Pietrzyk, and Thomas Sowa.
Lebek, S. , Wester, M. , Pec, J. , Poschenrieder, F. , Tafelmeier, M. , Fisser, C. , Provaznik, Z. , Schopka, S. , Debl, K. , Schmid, C. , Buchner, S. , Maier, L. S. , Arzt, M. , and Wagner, S. (2021) Abnormal P‐wave terminal force in lead V1 is a marker for atrial electrical dysfunction but not structural remodelling. ESC Heart Failure, 8: 4055–4066. 10.1002/ehf2.13488.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan G‐A, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau J‐P, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, van Gelder IC, van Putte BP, Watkins CL. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J 2020. Published online ahead of print 29 August 2020. [Google Scholar]
- 2. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D'Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim Y‐H, Lip GYH, Ma C‐S, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, van Wagoner DR, Nattel S. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterisation, and clinical implication. J Arrhythm 2016; 32: 247–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Overvad TF, Nielsen PB, Larsen TB, Søgaard P. Left atrial size and risk of stroke in patients in sinus rhythm. A systematic review. Thromb Haemost 2016; 116: 206–219. [DOI] [PubMed] [Google Scholar]
- 4. Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014; 63: 493–505. [DOI] [PubMed] [Google Scholar]
- 5. Liu G, Tamura A, Torigoe K, Kawano Y, Shinozaki K, Kotoku M, Kadota J. Abnormal P‐wave terminal force in lead V1 is associated with cardiac death or hospitalization for heart failure in prior myocardial infarction. Heart Vessels 2013; 28: 690–695. [DOI] [PubMed] [Google Scholar]
- 6. Kamel H, Healey JS. Cardioembolic stroke. Circ Res 2017; 120: 514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blume GG, Mcleod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PM, Tsang TSM. Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr 2011; 12: 421–430. [DOI] [PubMed] [Google Scholar]
- 8. Huber AT, Lamy J, Rahhal A, Evin M, Atassi F, Defrance C, Lebreton G, Clément K, Berthet M, Isnard R, Leprince P, Cluzel P, Hatem SN, Kachenoura N, Redheuil A. Cardiac MR strain: a noninvasive biomarker of fibrofatty remodeling of the left atrial myocardium. Radiology 2018; 286: 83–92. [DOI] [PubMed] [Google Scholar]
- 9. Huang Z, Zheng Z, Wu B, Tang L, Xie X, Dong R, Luo Y, Li S, Zhu J, Liu J. Predictive value of P wave terminal force in lead V1 for atrial fibrillation: a meta‐analysis. Ann Noninvasive Electrocardiol 2020; 25: e12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamel H, Bartz TM, Elkind MSV, Okin PM, Thacker EL, Patton KK, Stein PK, deFilippi CR, Gottesman RF, Heckbert SR, Kronmal RA, Soliman EZ, Longstreth WT. Atrial cardiopathy and the risk of ischemic stroke in the CHS (Cardiovascular Health Study). Stroke 2018; 49: 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamel H, Longstreth WT, Tirschwell DL, Kronmal RA, Broderick JP, Palesch YY, Meinzer C, Dillon C, Ewing I, Spilker JA, Di Tullio MR, Hod EA, Soliman EZ, Chaturvedi S, Moy CS, Janis S, Elkind MS. The AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke randomized trial: rationale and methods. Int J Stroke 2019; 14: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lebek S, Pichler K, Reuthner K, Trum M, Tafelmeier M, Mustroph J, Camboni D, Rupprecht L, Schmid C, Maier LS, Arzt M, Wagner S. Enhanced CaMKII‐dependent late INa induces atrial proarrhythmic activity in patients with sleep‐disordered breathing. Circ Res 2020; 126: 603–615. [DOI] [PubMed] [Google Scholar]
- 13. Lebek S, Plößl A, Baier M, Mustroph J, Tarnowski D, Lücht CM, Schopka S, Floerchinger B, Schmid C, Zausig Y, Pagratis N, Marchand B, Koltun DO, Hung WK, Ahmadyar S, Belardinelli L, Maier LS, Wagner S. The novel CaMKII inhibitor GS‐680 reduces diastolic SR Ca leak and prevents CaMKII‐dependent pro‐arrhythmic activity. J Mol Cell Cardiol 2018; 118: 159–168. [DOI] [PubMed] [Google Scholar]
- 14. Fischer TH, Neef S, Maier LS. The Ca‐calmodulin dependent kinase II: a promising target for future antiarrhythmic therapies? J Mol Cell Cardiol 2013; 58: 182–187. [DOI] [PubMed] [Google Scholar]
- 15. Buchner S, Greimel T, Hetzenecker A, Luchner A, Hamer OW, Debl K, Poschenrieder F, Fellner C, Riegger GAJ, Pfeifer M, Arzt M. Natural course of sleep‐disordered breathing after acute myocardial infarction. Eur Respir J 2012; 40: 1173–1179. [DOI] [PubMed] [Google Scholar]
- 16. Altmann U, Böger CA, Farkas S, Mack M, Luchner A, Hamer OW, Zeman F, Debl K, Fellner C, Jungbauer C, Banas B, Buchner S. Effects of reduced kidney function because of living kidney donation on left ventricular mass. Hypertension 2017; 69: 297–303. [DOI] [PubMed] [Google Scholar]
- 17. Tafelmeier M, Knapp M, Lebek S, Floerchinger B, Camboni D, Wittmann S, Creutzenberg M, Zeman F, Schmid C, Maier LS, Wagner S, Arzt M. Rationale and design of the CONSIDER AF study. Somnologie 2019; 23: 17–28. [Google Scholar]
- 18. Kwon Y, Misialek JR, Duprez D, Alonso A, Jacobs DR, Heckbert SR, Redline S, Soliman EZ. Association between sleep disordered breathing and electrocardiographic markers of atrial abnormalities: the MESA study. Europace 2017; 19: 1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scatteia A, Baritussio A, Bucciarelli‐Ducci C. Strain imaging using cardiac magnetic resonance. Heart Fail Rev 2017; 22: 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dick A, Schmidt B, Michels G, Bunck AC, Maintz D, Baeßler B. Left and right atrial feature tracking in acute myocarditis: a feasibility study. Eur J Radiol 2017; 89: 72–80. [DOI] [PubMed] [Google Scholar]
- 21. Kowallick JT, Kutty S, Edelmann F, Chiribiri A, Villa A, Steinmetz M, Sohns JM, Staab W, Bettencourt N, Unterberg‐Buchwald C, Hasenfuß G, Lotz J, Schuster A. Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson 2014; 16: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Backhaus SJ, Stiermaier T, Lange T, Chiribiri A, Uhlig J, Freund A, Kowallick JT, Gertz RJ, Bigalke B, Villa A, Lotz J, Hasenfuß G, Thiele H, Eitel I, Schuster A. Atrial mechanics and their prognostic impact in Takotsubo syndrome: a cardiovascular magnetic resonance imaging study. Eur Heart J Cardiovasc Imaging 2019; 20: 1059–1069. [DOI] [PubMed] [Google Scholar]
- 23. Amzulescu MS, de Craene M, Langet H, Pasquet A, Vancraeynest D, Pouleur AC, Vanoverschelde JL, Gerber BL. Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging 2019; 20: 605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inoue YY, Alissa A, Khurram IM, Fukumoto K, Habibi M, Venkatesh BA, Zimmerman SL, Nazarian S, Berger RD, Calkins H, Lima JA, Ashikaga H. Quantitative tissue‐tracking cardiac magnetic resonance (CMR) of left atrial deformation and the risk of stroke in patients with atrial fibrillation. J Am Heart Assoc 2015; 4: e001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alpert MA, Munuswamy K. Electrocardiographic diagnosis of left atrial enlargement. Arch Intern Med 1989; 149: 1161–1165. [PubMed] [Google Scholar]
- 26. Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII‐dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res 2010; 106: 1134–1144. [DOI] [PubMed] [Google Scholar]
- 27. Petersson R, Berge HM, Gjerdalen GF, Carlson J, Holmqvist F, Steine K, Platonov PG. P‐wave morphology is unaffected by atrial size: a study in healthy athletes. Ann Noninvasive Electrocardiol 2014; 19: 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tiffany Win T, Ambale Venkatesh B, Volpe GJ, Mewton N, Rizzi P, Sharma RK, Strauss DG, Lima JA, Tereshchenko LG. Associations of electrocardiographic P‐wave characteristics with left atrial function, and diffuse left ventricular fibrosis defined by cardiac magnetic resonance: the PRIMERI Study. Heart Rhythm 2015; 12: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rivaud MR, Marchal GA, Wolswinkel R, Jansen JA, van der Made I, Beekman L, Ruiz‐Villalba A, Baartscheer A, Rajamani S, Belardinelli L, van Veen TAB, Basso C, Thiene G, Creemers EE, Bezzina CR, Remme CA. Functional modulation of atrio‐ventricular conduction by enhanced late sodium current and calcium‐dependent mechanisms in Scn5a1798insD/+ mice. Europace 2020; 22: 1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study flowchart for patients with acute MI.
Figure S2. Study flowchart for patients undergoing elective CABG.
Figure S3. Strain rate values for kidney donors and patients with acute MI.
Figure S4. Trabecular fibrosis alters electrical conduction.
Table S1: Baseline characteristics of all patients with CMR data.
Table S2: Atrial strain from CMR.
Table S3: Univariate linear regression for the magnitude of PTFV1.
Table S4: Baseline characteristics of patients undergoing CABG.
Table S5: Abnormal PTFV1 as an independent predictor for the severity of trabecular arrhythmias.
Table S6: Abnormal PTFV1 is negatively and independently associated with atrial fibrosis.


