Abstract
Aims
This study aimed to investigate the association between the ‘Shrunken pore syndrome’ (SPS) and risk of death, 30 day rehospitalization, and health‐related quality of life (QoL) in heart failure (HF) patients. SPS is characterized by a difference in renal filtration between cystatin C and creatinine, resulting in a low eGFRcystatin C/eGFRcreatinine ratio.
Methods and results
A total of 373 patients hospitalized for HF [mean age 74.8 (±12.1) years; 118 (31.6%) women] were retrieved from the HeARt and brain failure inVESTigation trial (HARVEST‐Malmö). Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) formulas were used for estimation of glomerular filtration rate (eGFR). Presence of SPS was defined as eGFRcystatin C ≤ 60% of eGFRcreatinine. In Cox regression multivariate models, associations between SPS, risk of death (median follow‐up time 1.8 years), and risk of 30 day rehospitalization were studied. Associations between SPS and impaired QoL were studied using multivariate logistic regressions. In multivariate models, SPS was associated with all‐cause mortality [124 events; hazard ratio (HR) 1.99; 95% confidence interval (95% CI) 1.23–3.21; P = 0.005] and with 30 day rehospitalization (70 events; HR 1.82; CI 95% 1.04–3.18; P = 0.036). Analyses of QoL, based on a Kansas City Cardiomyopathy Questionnaire overall score < 50, revealed that SPS was associated with higher risk of low health‐related QoL (odds ratios 2.15; CI 95% 1.03–4.49; P = 0.042).
Conclusions
The results of this observational study show for the first time an association between SPS and poor prognosis in HF. Further studies are needed to confirm the results in HF cohorts and experimental settings to identify pathophysiological mechanisms.
Keywords: Cardiorenal syndrome, Creatinine, Cystatin C, Mortality, Quality of life, Shrunken pore
Introduction
Cardiovascular disease (CVD) [including heart failure (HF)] is the most common cause of death in patients with chronic kidney disease (CKD), 1 and there is vast knowledge about the association between CKD and CVD. The close relationship between heart disease and kidney disease is described as the cardiorenal syndrome (CRS). 2 Decreased renal function measured from plasma creatinine has been associated with increased risk for CVD morbidity and mortality, 1 but plasma cystatin C is associated with an even greater risk. 3 Corresponding results have been reported for estimation of glomerular filtration rate (eGFR) based upon creatinine 4 or cystatin C. 5 Although cystatin C is a more accurate marker of GFR, 6 it has not been clearly shown that its ability to measure renal function is the reason for cystatin C being a better marker for CVD risk. 7 On the other hand, data from Svensson‐Färbom and co‐workers reported that genetic elevation of plasma cystatin C was not related to altered risk of coronary artery disease (CAD), supporting the notion that there is no causal relationship between plasma cystatin C and CAD. Rather, the association between cystatin C and CAD appeared to be due to the association of renal dysfunction and CAD. 8
Several mechanisms have been proposed to explain the pathophysiology behind the CRS 9 , 10 : hypertension via the renin‐angiotensin‐aldosterone (RAAS) system, resulting in sodium overload and left ventricular hypertrophy; activation of RAAS that may lead to cardiac remodelling and myocardial fibrosis; and elevation in central venous pressure that lead to lower renal perfusion. Also, many neurohormonal and inflammatory mechanisms are implicated in the progression of CRS. These include increased formation of reactive oxygen species, arginine vasopressin, and endothelin, as well as excessive sympathetic activity that can result in myocardial hypertrophy and necrosis, damage to the microcirculations in kidney, glomerular sclerosis, and further stimulations of RAAS. 9 , 10 , 11
Recently, a new hypothesis, first presented by Grubb et al. in 2015, has emerged. It is based on difference in the glomerular filtration of small molecules (<0.2 kDa), for example, water and creatinine, vs. medium‐sized molecules of 5–40 kDa, for example, cystatin C and beta‐2 microglobulin, 12 over the glomerular filtration barrier, introduced as the ‘Shrunken pore syndrome’ (SPS) (Figure 1 ). 13 However, a decreased pore size is not the only possible mechanism leading to SPS and/or reduced eGFR from cystatin C vs. creatinine. Recently, our group showed that thickening of the glomerular basal membrane, and thus increasing the diffusion length of cystatin C, lowers the eGFRcystatin C/eGFRcreatinine ratio in kidney biopsies of subjects with diabetic nephropathy. 14
Figure 1.
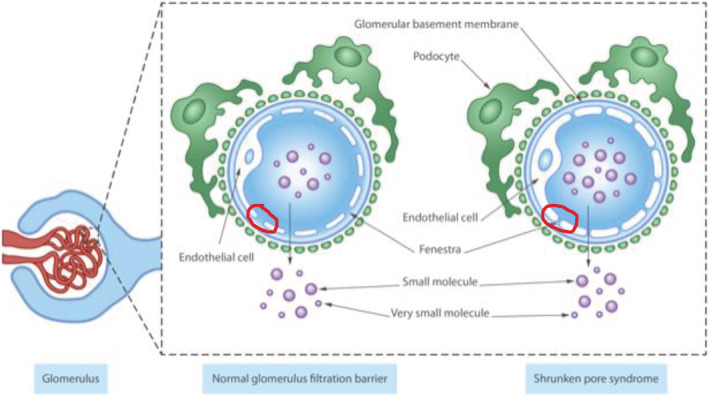
Schematic view of possible pathophysiology of Shrunken pore syndrome showing that fenestra between endothelial cells becomes narrower and the concentrations of middle‐sized molecules in plasma are higher.
Emerging data demonstrates a high risk for morbidity and mortality in CVD 15 , 16 , 17 , 18 for patients with SPS, also regardless of GFR level. 16 However, although we previously reported a cross‐sectional association between SPS and prevalent right ventricular HF, 19 to date, no studies have examined the impact of SPS on prognosis (death and rehospitalization) and quality of life (QoL) in an HF patient setting. Congestive HF is a major contributor to CKD, and contrariwise, CKD is a major contributor to cardiac damage. 20 Therefore, the aim of the present study was to investigate the association of SPS and mortality, morbidity, and QoL in an acute congestive HF cohort.
Methods
Study population
The Swedish Heart and Brain Failure Investigation Study (HARVEST‐Malmö) is an ongoing, prospective study undertaken in consecutive patients hospitalized for newly diagnosed or exacerbated acute heart failure (AHF), at the Skåne University Hospital in Malmö, Sweden. The only exclusion criterion was failure to obtain informed consent. In the case of patients with severe cognitive impairment, the consent was given by patient's relatives. 21 Baseline data including blood sample donations and clinical examination were collected between March 2014 and January 2019 in 411 subjects. Complete data on all variables were available in 373 subjects. Median follow‐up time for total mortality was 1.8 years. Mortality data were collected from the population register run by the Swedish Tax Agency in January 2019.
Clinical examination
Upon hospitalization, fasting blood samples were drawn (the same day or the day after), blood pressure was measured (mmHg), and body mass index (BMI) was calculated as kilograms per square metre. Subjects' health status (symptoms, function, and QoL) was evaluated using Kansas City Cardiomyopathy Questionnaire (KCCQ), a valid and reliable measure of health status in both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF), 22 also validated in Swedish. 23 An overall summary score < 50 of KCCQ was considered as an indication of low health‐related QoL, whereas overall summary scores ≥ 50 indicate a better health‐related QoL. 24 Prevalent diabetes mellitus (DM) was defined as prior physician's diagnosis of type 1 or type 2 diabetes or use of antidiabetic medication. Ischaemic heart disease (IHD) was defined as physician's diagnosis of myocardial infarction or angina pectoris, treatment with percutaneous coronary intervention or coronary artery bypass grafting, pathological myocardial perfusion imaging, pathological exercise electrocardiogram, or pathological coronary angiogram. Atrial fibrillation (AF) was defined as a pre‐hospitalization diagnosis of AF, or prevalent AF at electrocardiogram at hospitalization. Hypertension (HT) was defined as prior physician's diagnosis of HT, use of anti‐HT medication, or at least three measurements of systolic blood pressure (SBP) > 140 mmHg and/or diastolic blood pressure (DBP) > 90 mmHg. Baseline SBP and DBP were obtained after 10 min of rest in the supine position. A validated automated BP monitor (Boso Medicus, Bosch + Sohn GmbH u. Co. KG, Jungingen, Germany) was used. The upper arm cuff of appropriate size was placed on the right side, and the arm was supported at the heart level. Two measurements were performed with an interval of 30 s, and the mean value was calculated. Ethnicity was self‐reported and defined as Nordic or non‐Nordic ethnicity (European or non‐European descent). Employment status was self‐reported and defined as employed or non‐employed, retired for health reasons, or retired for non‐health reasons. Smoking status was self‐reported in a questionnaire (current smoker yes/no). Information on medication was collected using patients' electronical medical charts.
Laboratory assays and glomerular filtration rate estimations
Measurements of total cholesterol, N‐terminal prohormone BNP (NT‐proBNP), creatinine, and cystatin C were carried out at the Department of Clinical Chemistry, Skåne University Hospital in Malmö, participating in a national standardization and quality control system (EQUALIS). Plasma creatinine was measured using an enzymatic colorimetric assay with an IDMS‐traceable calibrator on the Hitachi Modular P analysis system (Roche, Basel, Switzerland). 25 The total analytical imprecision was 3.0% (with a concentration of 60 μmol/L in control sample) and 1.4% (with a concentration of 578 μmol/L in control sample; normal reference range: 60–105 μmol/L for men and 50–90 μmol/L for women). The plasma level of cystatin C was determined by an automated particle‐based immunoassay, adjusted to the international reference preparation ERM‐DA 471/IFCC, 26 using the Hitachi Modular P analysis system and reagents from DAKO (Dako A/S, Glostrup, Denmark). The total analytical imprecision was 2.1% (with concentration of 1.0 mg/L in control sample) and 1.7% (with concentration of 4.0 mg/L in control sample). NT‐proBNP was analysed using a sandwich assay based on ElectroChemiLuminiscence Immunoassay (Cobas, Roche Diagnostic, Basel, Switzerland).
Formulas for estimating glomerular filtration rate
CKD‐EPIcystatin C and CKD‐EPIcreatinine 27 estimating equations, based on cystatin C and creatinine, respectively, were used to estimate GFR. SPS was defined as eGFRcystatin C ≤ 60% of eGFRcreatinine.
Calculations of Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) eGFRcreatinine and CKD‐EPI eGFRcystatin C were calculated as follows.
Chronic Kidney Disease Epidemiology Collaboration eGFRcreatinine
Chronic Kidney Disease Epidemiology Collaboration eGFRcystatin C
Echocardiography
All studies were performed by experienced sonographers. We used Philips IE333 with a 1–5 MHz transducer, or GE Vingmed Vivid 7 Ultrasound with a 1–4 MHz transducer for transthoracic echocardiograms (TTE). Standard viewer (parasternal long axis, apical four‐chamber, and two‐chamber) was used to obtained cine loops. Left ventricular ejection fraction (LVEF) was calculated automatically from end‐diastolic volumes (EDV) and end‐systolic volume (ESV) (EF = (EDV − ESV)/EDV). A complete description is given elsewhere. 19
Endpoints
All‐cause mortality was identified through record linkage of the 10‐digit personal identification number of each Swedish citizen with the Swedish Cause of Death Register (SCDR) until January 2019. Median follow‐up time for mortality was 1.8 years. Data regarding 30 day rehospitalization (first of any) due to cardiac causes were retrieved by electronical medical charts (Melior, Siemens).
Statistical analyses
IBM SPSS statistics Version 25 (SPSS Inc, Chicago, IL) was used for all analyses. Baseline (admission) data are presented as mean with standard deviation (SD), median (25th–75th interquartile range), or as absolute numbers with percentages. For continuous variables, one‐way ANOVA was used to evaluate any significant differences (P < 0.05) in all the parameters between groups of eGFRcystatin C/eGFRcreatinine ratio; for binary variables, χ 2 tests were carried out. Cox regression models adjusted for age and sex were carried out (Model 1), followed by Cox regression multivariate adjusted models to determine associations between SPS and risk of all‐cause mortality (Model 2a) and risk of 30 day rehospitalization (Model 2b). Model 2a was adjusted for age, sex, BMI, AF, smoking status, SBP at admission, DM, IHD, log‐transformed NT‐proBNP, total cholesterol, and New York Heart Association (NYHA) class at admission. Further, eGFRcreatinine and LVEF were entered separately on top of Model 2a. Model 2b was adjusted for age, sex, DM, ethnicity, employment status, NYHA class at admission, log‐transformed NT‐proBNP, HDL, and SBP at admission. Further, subgroup analyses for associations between SPS and mortality/30 day rehospitalization were carried out using Cox regression model in subgroups of (a) LVEF ≤ 35% (HFrEF), (b) LVEF > 35 but ≤50% [HF with mid‐regional ejection fraction (HFmrEF)], and (c) LVEF > 50% (HFpEF) adjusted according to Model 1, and Models 2a and 2b, respectively. Logistic regression analyses were carried out for associations between SPS and low QoL (KCCQ < 50) adjusted for age and sex (Model 1) and further adjusted according to Model 2b.
Ethical approval
The study complies with the Declaration of Helsinki and was approved by the Ethical Review Board at Lund University, Sweden. A written informed consent was obtained from all patients or their relatives (Dnr 2013/360).
Results
The patient cohort was split in two different categories according to the ratios between eGFRcystatin C and eGFRcreatinine, and patient characteristics are presented in Table 1 . The mean age in the whole cohort was 75 years (SD ± 12.1). Most patients (99%) were treated with angiotensin‐converting enzyme inhibitor and/or angiotensin II receptor antagonist drugs. Linear regression ANOVA at baseline showed significant differences (P < 0.05) for sex, smoking, aldosterone antagonists, NYHA class, HDL, LVEF, creatinine, cystatin C, eGFRcystatin C, and eGFRcreatinine. SPS was more common in women, smokers, and subjects with longer HF duration (as opposed to new‐onset HF). Patients with SPS used less aldosterone antagonists. In contrast to patients without SPS were younger and had a lower NYHA class and LVEF (Table 1 ). Correlations between the eGFRcystatin C/eGFRcreatinine ratio and other measures of kidney function are presented in Supporting Information, Figure S1 .
Table 1.
Baseline characteristics of the study population
| Total n = 373 | eGFR ratio ≤ 0.6 n = 94 | eGFR ratio > 0.6 n = 279 | P‐value | |
|---|---|---|---|---|
| Demography | ||||
| Age (years) | 74.8 (±12.1) | 77.4 (±11.1) | 74.0 (±12.3) | 0.017 |
| Sex (female) [n (%)] | 118 (31.6) | 63 (67.0) | 55 (19.7) | <0.001 |
| Ethnicity | 0.403 | |||
| Nordic [n (%)] | 330 (88.5) | 87 (92.6) | 243 (87.1) | |
| Non‐Nordic European [n (%)] | 36 (9.7) | 7 (7.4) | 29 (10.4) | |
| Non‐European [n (%)] | 6 (1.8) | — | 6 (2.2) | |
| Employment status | 0.062 | |||
| Employed | 17 (4.6) | 2 (2.1) | 15 (5.4) | |
| Non‐employed | 49 (13.1) | 6 (6.4) | 43 (15.4) | |
| Retired for health reasons | 13 (3.5) | 4 (4.3) | 9 (3.2) | |
| Retired for non‐health reasons | 294 (78.8) | 82 (87.2) | 212 (76.0) | |
| Clinical profile | ||||
| Smoking [n (%)] | 44 (11.8) | 18 (19.1) | 26 (9.3) | 0.011 |
| BMI (kg/m2) | 27.9 (±6.0) | 29.1 (±7.3) | 27.4 (±5.4) | 0.018 |
| New‐onset heart failure | 114 (30.5) | 17 (18.1) | 97 (34.7) | 0.002 |
| Diuretic dosage at discharge (mg, n = 307) | 60 (40–80) | 70 (40–120) | 60 (40–80) | 0.083 |
| SBP (mmHg) | 137 (±27) | 140.1 (±27.2) | 136.8 (±27.9) | 0.308 |
| Beta‐blockers [n (%)] | 329 (88.2) | 78 (83.06) | 251 (90.0) | 0.069 |
| ACEi or ARB [n (%)] | 298 (79.9) | 70 (74.50) | 228 (81.7) | 0.113 |
| Aldosterone antagonists [n (%)] | 24 (6.4) | 2 (2.1) | 22 (7.9) | 0.048 |
| Loop diuretics [n (%)] | 359 (96.2) | 92 (97.9) | 267 (95.7) | 0.338 |
| Diabetes [n (%)] | 136 (36.5) | 39 (41.5) | 97 (34.8) | 0.242 |
| NYHA class | 0.342 | |||
| I–II | 46 (12.4) | 7 (7.4) | 39 (14.0) | |
| III–IV | 327 (87.6) | 87 (92.6) | 240 (86.0) | |
| AF [n (%)] | 225 (60.3) | 58 (61.7) | 167 (59.9) | 0.752 |
| IHD [n (%)] | 145 (38.9) | 31 (33.0) | 114 (40.9) | 0.175 |
| Laboratory | ||||
| Total cholesterol (mmol/L) | 3.6 (±1.01) | 3.5 (±1.0) | 3.6 (±1.0) | 0.336 |
| Cystatin C (mg/L) | 1.8 (1.3–2.2) | 2.1 (1.6–2.5) | 1.7 (1.2–2.0) | <0.001 |
| Creatinine (mmol/L) | 105 (84–136) | 103 (79–124) | 120 (88–141) | 0.007 |
| eGFR (mL/min/1.73 m2)CKD‐EPI | 43.2 (±18.6) | 42.1 (±16.0) | 43.6 (±19.3) | 0.533 |
| eGFR CKD‐EPIcystatin C | 37.8 (±17.1) | 30.4 (±12.2) | 40.2 (±17.8) | <0.001 |
| e‐GFR CKD‐EPIcreatinine | 51.6 (±22.6) | 60.6 (±22.3) | 48.6 (±21.9) | <0.001 |
| CRP (mg/L) | 9.4 (4.9–22.0) | 12.0 (5.1–25.0) | 9.0 (4.8–21.0) | 0.355 |
| NT‐proBNP (ng/L) | 4141 (2237–8693) | 4189 (2380–9708) | 4141 (2178–8117) | 0.389 |
| Echocardiography | ||||
| n = 268 | n = 58 | n = 210 | ||
| LVEF (%) | 38.5 (±16.1) | 42.7 (±16.2) | 37.4 (15.9) | 0.026 |
| n = 244 | n = 191 | |||
| TAPSE (mm) | 16.6 (±5.4) | 17.2 (±5.3) | 16.6 (±5.0) | 0.426 |
ACEi, angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin II receptor antagonists; BMI, body mass index; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CRP, C‐reactive protein; GFR, glomerular filtration rate; HDL, high density lipoprotein; IHD, ischaemic heart disease; LDL, low density lipoprotein; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SBP, systolic blood pressure; TAPSE, tricuspidal annular plane systolic excussion.
Values are means (±standard deviation) or medians (25th–75th interquartile range).
Shrunken pore syndrome and risk of death
In total, 124 patients died during the follow‐up time. Hazard ratio (HR) for all‐cause mortality using Cox multivariate analysis comparing patients with SPS and those with an eGFRcystatin C/eGFRcreatinine ratio > 0.6 is shown in Table 2 .
Table 2.
Cox regression model of association between SPS based on the Chronic Kidney Disease Epidemiology Collaboration formulas (n = 94 of the total 373 subjects) and all‐cause mortality (124 events)
| HR | CI 95% | P | |
|---|---|---|---|
| SPS | 1.99 | (1.23–3.21) | 0.005 |
| Age | 1.06 | (1.04–1.09) | 4.69 × 10−8 |
| Sex | 0.37 | (0.23–0.61) | 8.89 × 10−5 |
| Smoking | 1.52 | (0.82–2.81) | 0.184 |
| Atrial fibrillation | 0.63 | (0.42–0.94) | 0.024 |
| Diabetes | 1.02 | (0.67–1.55) | 0.912 |
| BMI | 1.02 | (0.98–1.06) | 0.261 |
| NYHA class | |||
| I–II | 1.05 | (0.51–2.13) | 0.901 |
| ≥III | 1.57 | (0.96–2.56) | 0.074 |
| Systolic blood pressure | 0.99 | (0.98–1.00) | 0.003 |
| Total cholesterol | 0.90 | (0.73–1.11) | 0.343 |
| NT‐proBNP | 1.53 | (1.25–1.88) | 4.23 × 10−5 |
| Ischaemic heart disease | 0.72 | (0.48–1.08) | 0.114 |
BMI, body mass index; CI, confidence interval; HR, hazard ratio; NT‐proBNP, N‐terminal prohormone BNP; NYHA, New York Heart Association class at admission; SPS, Shrunken pore syndrome.
Hazard ratio for all‐cause mortality for patients with SPS compared with those with an eGFRcystatin C/eGFRcreatinine ratio > 0.6 was 2.14 [95% confidence interval (95% CI) 1.35–3.39] in Model 1 and 1.99 (CI 95% 1.23–3.21) in Model 2a. The association of SPS with outcome persisted when further adjusted for eGFRcreatinine (HR 1.90; CI 95% 1.18–3.07; P = 0.009) or for eGFR from the CKD‐EPI (HR 1.77; CI 95% 1.10–2.85; P = 0.019). Similarly, SPS remained significantly associated with the risk of all‐cause mortality when LVEF was entered upon Model 2a (n = 268; 83 events; HR 2.06; CI 95% 1.16–3.65; P = 0.014). Further, we carried out analyses adjusting for right ventricular function by entering tricuspidal annular plane systolic excussion (TAPSE) on top of Model 2a, with SPS still significantly associated with all‐cause mortality (HR 2.11; CI 95% 1.13–3.93; P = 0.019). Finally, we entered standardized diuretic dosage at discharge on top of Model 2a, with SPS remaining significantly associated with higher risk of death (n = 307; 120 events; HR 1.95; CI 95% 1.19–3.19; P = 0.008).
Mortality rates differed between the patients with SPS and those with eGFRcystatin C/eGFRcreatinine ratio > 0.6 already within the first 4 months of follow‐up and continued to increase during the follow‐up time as shown by unadjusted Kaplan–Meier survival curves (Figure 2A ). The same was true for cumulative hazard (Figure 2B ).
Figure 2.
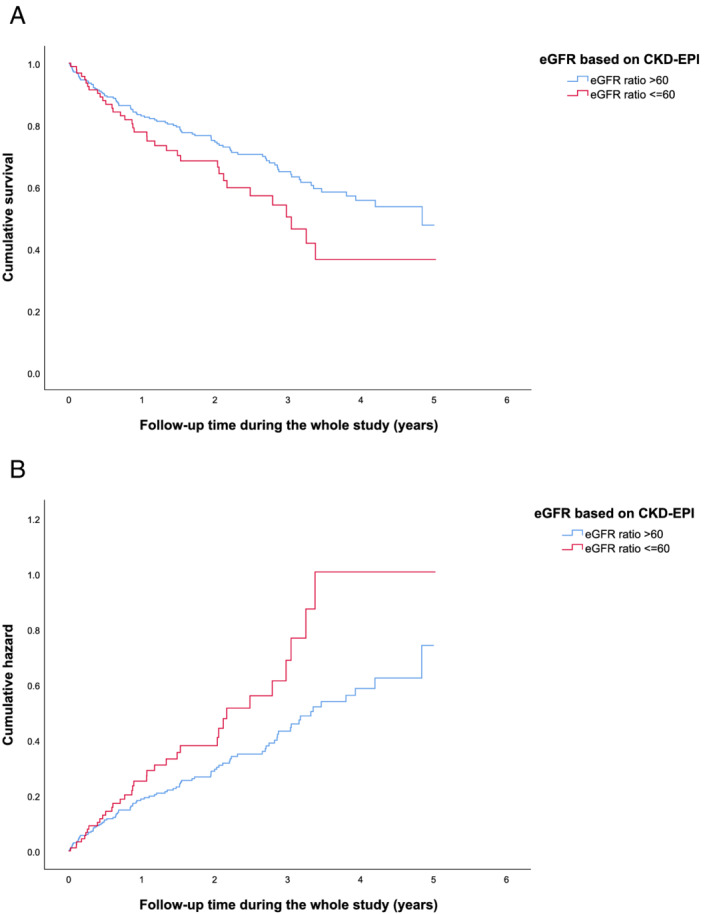
(A) Kaplan–Meier survival curves for patients with Shrunken pore syndrome (SPS) (eGFRcystatin C/eGFRcreatinine ratio ≤ 0.6) (red) and patients with an eGFRcystatin C/eGFRcreatinine ratio > 0.6 (blue), during the follow‐up time. SPS is calculated based on CKD‐EPI formulas. (B) Cumulative hazard for the patients with SPS (red) and patients with an eGFRcystatin C/eGFRcreatinine ratio > 0.6 (blue), during the follow‐up time. Calculations are based on CKD‐EPI formulas. CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimation of glomerular filtration rate.
Subgroup analyses
Analyses of associations between SPS and risk of death were carried out in subgroups of ‘HFrEF’, ‘HFmrEF’, and ‘HFpEF’. A total of 268 patients underwent echocardiography, and associations between SPS and mortality/30 day rehospitalization according to categories HFrEF, HFmrEF, and HFpEF are presented in Supporting Information, Table S1 . No association was seen between risk of death and SPS for subjects belonging to HFrEF, whereas SPS was associated with risk of death for subjects with HFmrEF and HFpEF (Supporting Information, Table S1 ).
Shrunken pore syndrome and 30 day rehospitalization risk
Shrunken pore syndrome was associated with higher risk of 30 day rehospitalization (70 events) in Model 2b (HR 1.82; CI 95% 1.04–3.18; P = 0.036) (Table 3 ). In subgroup analyses, SPS was associated with 30 day rehospitalization risk in subjects with HFpEF, but not in subjects with LVEF ≤ 50% (Supporting Information, Table S1 ).
Table 3.
Associations between SPS and 30 day rehospitalization, and lower quality of life
| Subjects with SPS based on the CKD‐EPI formulas (n = 98 of the total 373 subjects) | ||||
|---|---|---|---|---|
| 30 day rehospitalization (70 events) | KCCQ overall score < 50 points (n = 54) | |||
| HR (CI 95%) | P‐value | OR (CI 95%) | P‐value | |
| Model 1 | 1.73 (0.99–3.02) | 0.023 | 2.41 (1.21–4.81) | 0.012 |
| Model 2b | 1.82 (1.04–3.18) | 0.036 | 2.15 (1.03–4.49) | 0.042 |
CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimation of glomerular filtration rate; KCCQ, Kansas City Cardiomyopathy Questionnaire; SPS, Shrunken pore syndrome.
Patients with SPS were compared with those with an eGFRcystatin C/eGFRcreatinine ratio > 0.6.
Values are hazard ratios (HR) or odds ratios (OR) and 95% confidence intervals (CI 95%).
Model 1: adjusted for age and sex.
Model 2b: adjusted for age, sex, diabetes mellitus, ethnicity, employment, New York Heart Association class at admission, N‐terminal prohormone BNP, HDL, and systolic blood pressure at admission.
Shrunken pore syndrome and quality of life
Cross‐sectional analyses of QoL, based on a KCCQ overall score, revealed that SPS was significantly associated with increased risk of low health‐related QoL defined as an overall summary score < 50 [Table 3 ; odds ratios 2.41 in Model 1 and 2.15 (CI 95% 1.03–4.49; P = 0.042) in Model 2b].
Discussion
The main finding of this prospective study demonstrates, for the first time, that patients with HF and SPS exhibit an approximately doubled risk of all‐cause mortality and risk of 30 day rehospitalization when compared with HF patients without SPS. Furthermore, SPS contributed to a significantly impaired QoL in patients with HF.
Our results are in coherence with other studies carried out in other patient groups with CVD, 15 , 16 , 17 , 18 although this study is carried out in an AHF setting and, thus, reflects a congestive state, which in turn is associated with declined kidney function. 20 Dardashti et al. showed that SPS (defined as eGFRcystatin C ≤ 70% of eGFRcreatinine) was associated with a rise in mortality in patients undergoing elective coronary artery bypass grafting with an HR of nearly 3. 15 Lüders et al. studied the prevalence of acute kidney injury [contrast‐induced AKI (CI‐AK)] and the mortality and morbidity of the patients who underwent elective heart catheterization. They found that the pre‐interventional ratio of cystatin C–creatinine was independently associated with CI‐AKI and highly significantly associated with long‐term mortality after heart catheterization. 16 Purde et al. studied the prevalence of SPS (defined as eGFRcystatin C ≤ 60% of eGFRcreatinine) in an elderly population, and although the syndrome was prevalent at low rate (0.7%), the individuals with SPS had an increased rate of mortality and morbidity. 17 Herou et al. evaluated whether early and midterm mortality following elective cardiac surgery in 4000 patients varies with different cut‐off values for the ratio used to diagnose SPS. The authors found that mortality increased with decreasing ratios for eGFRcystatin C/eGFRcreatinine. The 1 and 3 year mortality was 10% and 21%, respectively, in patients with SPS defined as CKD‐EPI eGFRcystatin C ≤ 60% of eGFRcreatinine. Mortality at 1 and 3 year follow‐up was 6.6% and 14%, respectively, using 74% as the cut‐off value for identifying SPS. 18
Recently, in 2781 patients referred for iohexolclearance, Åkesson et al. showed that subjects with eGFRcystatin C/eGFRcreatinine ratio < 0.70 had a higher HR (3.0; CI 95% 2.4–3.7) for total mortality than cancer, CVD, DM, and CKD per se. 28 Among these patients, 1300 presented with a normal measured GFR (mGFR). Further, all‐cause mortality for subjects with eGFRcystatin C/eGFRcreatinine ratio < 0.70 was markedly increased (HR 4.1; CI 95% 2.6–6.5) compared with the subjects with eGFRcystatin C/eGFRcreatinine ratio > 1.
We used a valid and reliable measure for the estimation of QoL, the KCCQ, and could show a relationship between SPS and health‐related QoL. Further, we believe that our mortality data following hospitalization are in line with the general data for HF (10.4% at 30 days, 22% at 1 year, and 42.3% at 5 years). 29 Subgroup analyses revealed that SPS was associated with mortality and rehospitalization risk in subjects with HFmrEF and HFpEF, but not in subjects with LVEF ≤ 35%. This is possibly due to different mechanisms of death and rehospitalization in HFrEF vs. HFpEF. In HFpEF and HFmrEF, non‐cardiac death is a major determinant of outcome, exceeding cardiac‐related mortality, 30 while cardiac arrest is the mode of demise in 30–50% of patients with HFrEF, 31 which possibly overrides the higher risk imposed by concomitant renal dysfunction (or other co‐morbidities).
Diuretics have been shown to thicken the glomerular basal membrane, possibly resulting in diuretic resistance. 32 , 33 However, here, we found SPS to be independently associated with increased risk of death even after adjustment for diuretic resistance measured as diuretics dosage at discharge. Further, no significant association was found between SPS and diuretic resistance, further strengthening the hypothesis that the creatinine/cystatin C mismatch is due to separate mechanisms. Also, as illustrated in Supporting Information, Figure S1 , the correlations between eGFRcystatin C/eGFRcreatinine ratio and other measures of kidney function are weak, further strengthening the notion that SPS goes beyond conventional markers of kidney function such as eGFR.
It is not yet determined what causes the difference in the glomerular filtration of medium‐sized proteins (cystatin C, beta‐2‐microglobulin, etc.) and neither the mechanism behind the relation to cardiac diseases and mortality. It has recently been shown that in parallel with the increase of cystatin C and beta‐2‐microglobulin in SPS, there is also an increase in several 5–40 kDa cytokines known to promote atherosclerosis. 34 The genes for these proteins are located at different chromosomes and have different regulation elements, and production of these proteins is not co‐regulated and thus cannot explain the concordant increases of their plasma levels.
One hypothesis is that endothelial damages, common for the vasculature in the kidneys and the heart, may be responsible for the common pathology. A shrinking pore diameter in the glomerular filtration barrier as a consequence of endothelial damage may cause lower clearance for medium‐sized molecules, like cystatin C, compared with small molecules as creatinine. If so, this may explain the stronger association of cystatin C to CVD mortality than the association of creatinine to CVD that Shlipak et al. showed. 3 Our results showing strong associations of SPS with increased risk of mortality and morbidity as well as impaired QoL in HF patients further strengthen the hypothesis that SPS is an independent marker of heart disease in patients with CKD.
Strengths and limitations
There are both strengths and limitations to this study. As we included patients admitted for new or worsening HF, with inability to deliver informed consent to the study as the only exclusion criterion, our study population is most likely representative of an actual HF population. However, our data were collected at a single regional centre and the sample size was relatively small (n = 373), which limits the applicability to other populations of HF patients. The subjects included in HARVEST‐Malmö were mainly of European descent, and the conclusions drawn might not be generalizable to all ancestries. The patients in our study have a low use of aldosterone antagonists, which can explain the relatively high rate of mortality.
There were no data available on urinary albumin excretion, which could have been used as an alternative prognostic marker, or in combination with SPS.
Cystatin C levels were higher in patients with SPS, which results in a lower eGFRcystatin C, but the combined formula with both creatinine and cystatin C was similar in those with and without SPS. This is typical for patients with SPS. Findings of a thicker glomerular basement membrane in patients with SPS in the study by Öberg et al. may explain the lower clearance of medium‐sized molecules such as cystatin C, compared with small molecules like creatinine. 14 This difference in clearance for medium‐sized and small molecules explains the changes in plasma levels of cystatin C and creatinine. As BMI was higher in the group with SPS, the higher eGFRcreatinine cannot be explained by a lower muscle mass; otherwise, this could have been an explanation. However, we have no data on specific determination of muscle mass in this cohort, nor any information if the patients were on treatment with high doses of corticosteroids, which can alter the plasma levels of cystatin C.
At the point the analyses were carried out, we had analysed echocardiography data on only 268 of 373 patients. Although adjusting for LVEF did not change the fact that SPS was significantly associated with rise in mortality, we cannot exclude that the results could have been attenuated if complete data were available. Further, we did not have discharge data on congestion status.
Although we attempted adjustment for a heterogeneous and clinically relevant panel of risk factors, the observational nature of this study prevents us from ruling out that residual confounders may have affected the outcome of our analysis.
Conclusions
The results of this prospective study show for the first time a relationship between SPS and poor prognosis in an HF setting. Further studies are needed to confirm the results in population‐based cohorts and experimental settings to reveal pathophysiological mechanisms as a basis for potential interventions.
Conflict of interest
The authors have no competing interests.
Funding
Dr Magnusson was supported by grants from the Medical Faculty of Lund University (Medicinska Fakulteten, Lunds Universitet), Skåne University Hospital (Skånes universitetssjukhus), the Crafoord Foundation (Crafoordska Stiftelsen), the Ernhold Lundstrom's Research Foundation (Ernhold Lundström Stiftelse), the Region Skåne (Hjärt‐Lungfonden), the Hulda and Conrad Mossfelt Foundation (Hulda och E Conrad Mossfelts Stiftelse för Vetenskaplig Forskning Inom Hjärt‐ och Kärlsjukdomarnas Område), the Southwest Skåne's Diabetes Foundation (Sydvästra Skånes Diabetesförening), the Kockska Foundation, the Research Funds of Region Skåne, the Swedish Heart and Lung Foundation, and the Wallenberg Center for Molecular Medicine, Lund University. Dr Christensson was supported by grants from the Medical Faculty of Lund University, the Swedish Kidney Foundation (Njurfonden), Njurstiftelsen, Skåne University Hospital Research Fund, and the Research and Development Council of Region Skåne (Skåne County Council's Research and Development Foundation), Sweden. Dr Jujic was supported by grants from Region Skåne and Lund University (Lunds Universitet).
Author contributions
L.X., M.M., A.C., A.G., and A.J. performed the research; M.M. and E.B. designed the research study; L.X., M.M., and A.J. analysed the data; L.X., A.J., M.M., and A.C. wrote the paper; all authors discussed the results and contributed to the final manuscript; and all authors have participated in drafting the article or revising it critically for important intellectual content.
Supporting information
Table S1. Cox regression models of association between SPS and mortality and 30‐day re‐hospitalization.
Figure S1. Correlations between eGFRcystatinC/eGFRcreatinine and other measures of kidney function.
Acknowledgements
We thank the research nurses Hjördis Jernhed and Dina Chatziapostolou for valuable contributions, and we thank all the staff at the echocardiographic laboratory at Skåne University Hospital Malmö. The Knut and Alice Wallenberg Foundation is acknowledged for generous support. The Fulbright Commission has stimulated building a strong research network. We thank the statistician Anna Åkesson for her input in our calculations.
Xhakollari, L. , Grubb, A. , Jujic, A. , Bachus, E. , Nilsson, P. M. , Leosdottir, M. , Christensson, A. , and Magnusson, M. (2021) The Shrunken pore syndrome is associated with poor prognosis and lower quality of life in heart failure patients: the HARVEST‐Malmö study. ESC Heart Failure, 8: 3577–3586. 10.1002/ehf2.13485.
References
- 1. Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32: S112–S119. [DOI] [PubMed] [Google Scholar]
- 2. Ronco C, House AA, Haapio M. Cardiorenal syndrome: refining the definition of a complex symbiosis gone wrong. Intensive Care Med 2008; 34: 957–962. [DOI] [PubMed] [Google Scholar]
- 3. Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman‐Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005; 352: 2049–2060. [DOI] [PubMed] [Google Scholar]
- 4. Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol 2000; 35: 1628–1637. [DOI] [PubMed] [Google Scholar]
- 5. Sarnak MJ, Katz R, Stehman‐Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG, and the Cardiovascular Health Study* . Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med 2005; 142: 497–505. [DOI] [PubMed] [Google Scholar]
- 6. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta‐analysis. Am J Kidney Dis 2002; 40: 221–226. [DOI] [PubMed] [Google Scholar]
- 7. Sundin PO, Sjostrom P, Jones I, Olsson LA, Udumyan R, Grubb A, Lindström V, Montgomery S. Measured glomerular filtration rate does not improve prediction of mortality by cystatin C and creatinine. Nephrol Dial Transplant 2017; 32: 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Svensson‐Färbom P, Almgren P, Hedblad B, Engström G, Persson M, Christensson A, Melander O. Cystatin C is not causally related to coronary artery disease. PLOS ONE 2015; 10: e0129269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Viswanathan G, Gilbert S. The cardiorenal syndrome: making the connection. Int J Nephrol 2010; 2011: 283137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation 2010; 121: 2592–2600. [DOI] [PubMed] [Google Scholar]
- 11. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 2008; 52: 1527–1539. [DOI] [PubMed] [Google Scholar]
- 12. Grubb A, Lindström V, Jonsson M, Bäck S‐E, Åhlund T, Rippe B, Christensson A. Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: ‘Shrunken pore syndrome’. Scand J Clin Lab Invest 2015; 75: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grubb A. Shrunken pore syndrome—a common kidney disorder with high mortality. Diagnosis, prevalence, pathophysiology and treatment options. Clin Biochem 2020; 83: 12–20. [DOI] [PubMed] [Google Scholar]
- 14. Öberg CM, Lindström M, Grubb A, Christensson A. Potential relationship between eGFRcystatin C/eGFRcreatinine‐ratio and glomerular basement membrane thickness in diabetic kidney disease. medRxiv 2020. 2020.12.16.20248179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dardashti A, Nozohoor S, Grubb A, Bjursten H. Shrunken Pore Syndrome is associated with a sharp rise in mortality in patients undergoing elective coronary artery bypass grafting. Scand J Clin Lab Invest 2016; 76: 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lüders F, Meyborg M, Malyar N, Reinecke H. The preinterventional cystatin‐creatinine‐ratio: a prognostic marker for contrast medium‐induced acute kidney injury and long‐term all‐cause mortality. Nephron 2015; 131: 59–65. [DOI] [PubMed] [Google Scholar]
- 17. Purde MT, Nock S, Risch L, Medina Escobar P, Grebhardt C, Nydegger UE, Stanga Z, Risch M. Ratio of cystatin C and creatinine‐based estimates of the glomerular filtration rate predicts mortality in healthy seniors independent of kidney function. Scand J Clin Lab Invest 2016; 76: 341–343. [DOI] [PubMed] [Google Scholar]
- 18. Herou E, Dardashti A, Nozohoor S, Zindovic I, Ederoth P, Grubb A, Bjursten H. The mortality increase in cardiac surgery patients associated with shrunken pore syndrome correlates with the eGFRcystatin C/eGFRcreatinine‐ratio. Scand J Clin Lab Invest 2019; 79: 167–173. [DOI] [PubMed] [Google Scholar]
- 19. Christensson A, Grubb A, Molvin J, Holm H, Gransbo K, Tasevska‐Dinevska G, Bachus E, Jujic A, Magnusson M. The shrunken pore syndrome is associated with declined right ventricular systolic function in a heart failure population—the HARVEST study. Scand J Clin Lab Invest 2016; 76: 568–574. [DOI] [PubMed] [Google Scholar]
- 20. Silverberg D, Wexler D, Blum M, Schwartz D, Iaina A. The association between congestive heart failure and chronic renal disease. Curr Opin Nephrol Hypertens 2004; 13: 163–170. [DOI] [PubMed] [Google Scholar]
- 21. Ali A, Holm H, Molvin J, Bachus E, Tasevska‐Dinevska G, Fedorowski A, Jujic A, Magnusson M. Autonomic dysfunction is associated with cardiac remodelling in heart failure patients. ESC Heart Fail 2018; 5: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joseph SM, Novak E, Arnold SV, Jones PG, Khattak H, Platts AE, Dávila‐Román VG, Mann DL, Spertus JA. Comparable performance of the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail 2013; 6: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel H, Ekman I, Spertus JA, Wasserman SM, Persson LO. Psychometric properties of a Swedish version of the Kansas City Cardiomyopathy Questionnaire in a chronic heart failure population. Eur J Cardiovasc Nurs 2008; 7: 214–221. [DOI] [PubMed] [Google Scholar]
- 24. Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation 2004; 110: 546–551. [DOI] [PubMed] [Google Scholar]
- 25. Nyman U, Grubb A, Larsson A, Hansson LO, Flodin M, Nordin G, Lindström V, Björk J. The revised Lund‐Malmo GFR estimating equation outperforms MDRD and CKD‐EPI across GFR, age and BMI intervals in a large Swedish population. Clin Chem Lab Med 2014; 52: 815–824. [DOI] [PubMed] [Google Scholar]
- 26. Grubb A, Horio M, Hansson LO, Bjork J, Nyman U, Flodin M, Larsson A, Bökenkamp A, Yasuda Y, Blufpand H, Lindström V, Zegers I, Althaus H, Blirup‐Jensen S, Itoh Y, Sjöström P, Nordin G, Christensson A, Klima H, Sunde K, Hjort‐Christensen P, Armbruster D, Ferrero C. Generation of a new cystatin C‐based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem 2014; 60: 974–986. [DOI] [PubMed] [Google Scholar]
- 27. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, van Lente F, Zhang YL, Coresh J, Levey AS, CKD‐EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Åkesson A, Lindstrom V, Nyman U, Jonsson M, Abrahamson M, Christensson A, Björk J, Grubb A. Shrunken pore syndrome and mortality: a cohort study of patients with measured GFR and known comorbidities. Scand J Clin Lab Invest 2020: 1–12. [DOI] [PubMed] [Google Scholar]
- 29. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities Study). Am J Cardiol 2008; 101: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 30. Vergaro G, Ghionzoli N, Innocenti L, Taddei C, Giannoni A, Valleggi A, Borrelli C, Senni M, Passino C, Emdin M. Noncardiac versus cardiac mortality in heart failure with preserved, midrange, and reduced ejection fraction. J Am Heart Assoc 2019; 8: e013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Packer M. What causes sudden death in patients with chronic heart failure and a reduced ejection fraction? Eur Heart J 2020; 41: 1757–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ellison DH, Loffing J. Thiazide effects and adverse effects. Hypertension 2009; 54: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reungjui S, Hu H, Mu W, Roncal CA, Croker BP, Patel JM, Nakagawa T, Srinivas T, Byer K, Simoni J, Wesson D, Sitprija V, Johnson RJ. Thiazide‐induced subtle renal injury not observed in states of equivalent hypokalemia. Kidney Int 2007; 72: 1483–1492. [DOI] [PubMed] [Google Scholar]
- 34. Almén MS, Björk J, Nyman U, Lindström V, Jonsson M, Abrahamson M, Vestergren AS, Lindhe Ö, Franklin G, Christensson A, Grubb A. Shrunken Pore Syndrome is associated with increased levels of atherosclerosis‐promoting proteins. Kidney Int Rep 2018; 4: 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cox regression models of association between SPS and mortality and 30‐day re‐hospitalization.
Figure S1. Correlations between eGFRcystatinC/eGFRcreatinine and other measures of kidney function.


