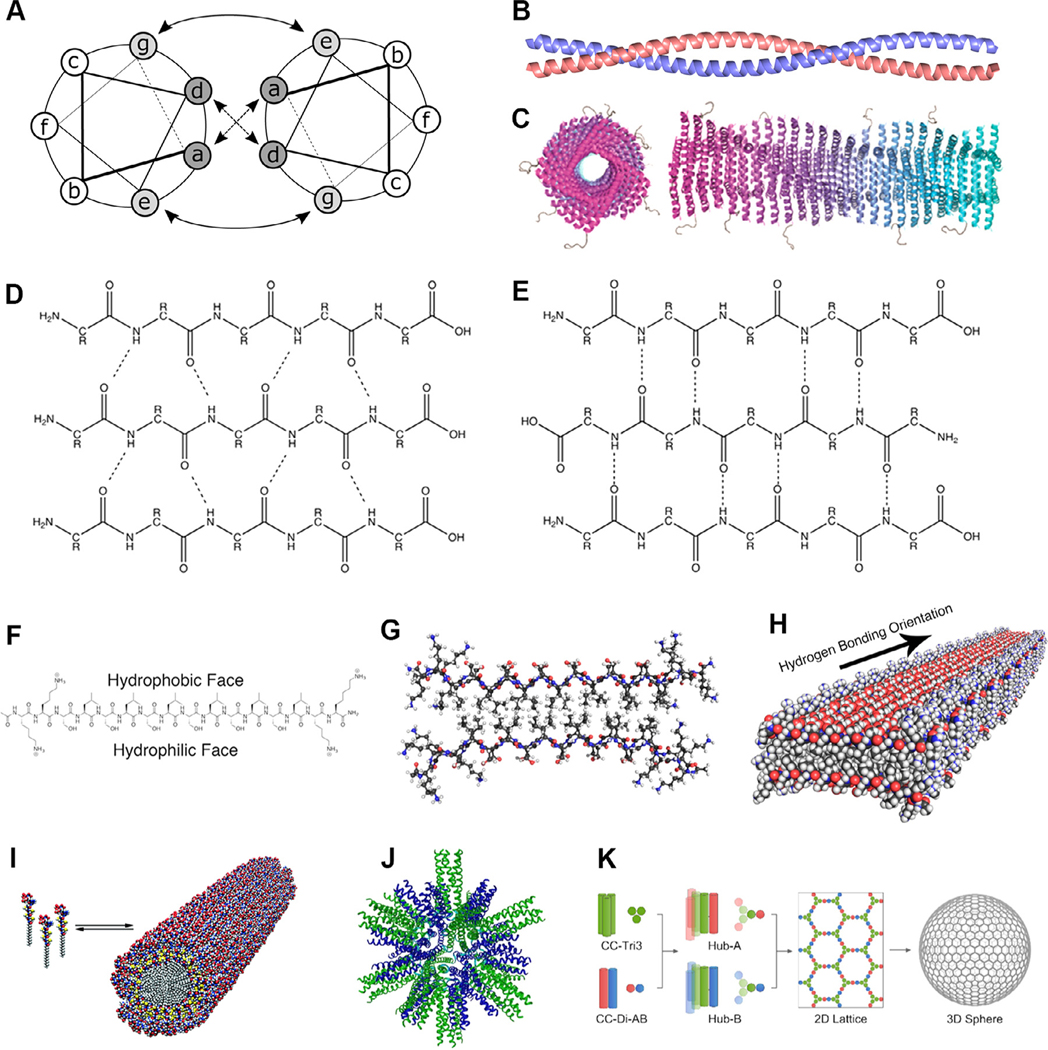
Fig. 1.
Supramolecular peptide, peptide amphiphile, and protein subunit nanoparticle structures. (A,B) Coiled coils are oligomers composed of two or more α-helices that typically display an abcdefg heptad repeat [33]. (C) Coil29 self-assembles into filamentous nanotubes in which individual coiled coil peptides associate with their N-termini facing radially outward (adapted with permission from [34], copyright 2017 American Chemical Society). β-sheets adopt either (D) parallel or (E) antiparallel orientation, both of which are stabilized by extensive hydrogen bonding networks [35]. β-sheets with alternating hydrophilic and hydrophobic residues are (F) facially amphipathic and (G) laminate into bilayers that (H) propagate along the hydrogen bonding axis (adapted with permission from [36], copyright 2017 American Chemical Society). (I) Peptide amphiphiles contain peptide head groups and lipid tails that assemble into cylindrical or spherical micelles (reprinted with permission from AAAS [37]). (J) Protein subunit nanoparticles incorporating trimeric and pentameric coiled coils assembly into polyhedral nanoparticles (adapted from [27], copyright 2006, with permission from Elsevier). (K) Coiled coil homotrimers covalently linked to the components of a heterodimeric coiled coil through disulfide bridges interact to form a hexagonal lattice that gives rise to closed nanocages (adapted with permission from [38], copyright 2018 American Chemical Society).