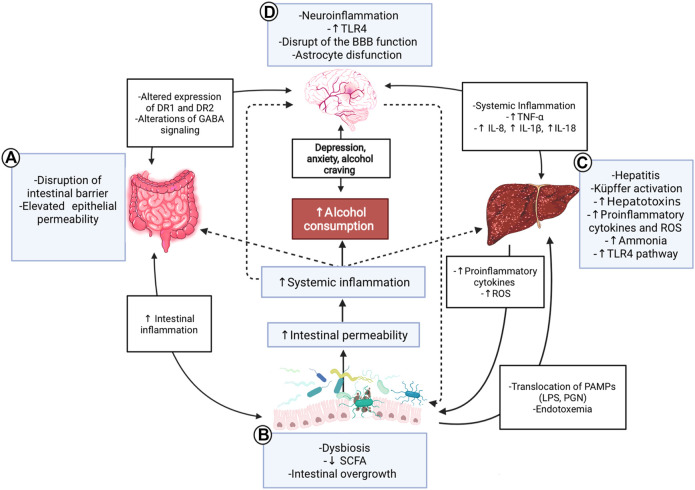
FIGURE 1.
Gut-microbiota-liver-brain axis in ALD. Interaction diagram of the different mechanisms participating in the gut-microbiota-liver-brain axis involved in the pathophysiology of ALD. (A) Alcohol consumption has adverse effects on the gut; it disrupts the gut barrier leading to high permeability and translocation of bacterial products. These effects create a proinflammatory environment which affects microbiota. (B) ALD has a specific microbiota dysbiosis favoring an overgrowth of nonbeneficial bacteria. The decrease of SCFA due to alcohol consumption influences these alterations because SCFA is food for helpful bacteria. This context produces a translocation of different substances called PAMPs, such as LPS or peptidoglycan, to the liver and circulation, increasing endotoxemia. (C) The liver is a vital organ in ethanol metabolization and suffers many changes in chronic consumption; activation of Küpffer cells and proinflammatory TLR4 pathway, causing hepatitis, increased reactive oxygen species, and cytokines, such as IL-18, IL-8, and IL-1β. In advanced stages, the liver fails in its detox task, and organisms accumulate ammonia. (D) All the aforementioned inflammatory processes lead to a systemic inflammation that affects the brain, contributing to ethanol-triggered neuroinflammation. PAMPs and alcohol also produce disruption of the blood-brain barrier, astrocyte senescence, and more significant changes in the brain; alteration of the DR1 and 2, increased levels of anxiety, depression, and alcohol craving. Finally, the gut and the microbiota are influenced by the brain and vice-versa through nerve and GABA signaling modulation. ALD: Alcoholic liver disease; SCFA: Short-chain fatty acids; PAMPs: Pathogen-associated molecular patterns; LPS: Lipopolysaccharide: PGN: Peptidoglycan; ROS: Reactive oxygen species; BBB: Blood-brain barrier; DR1/DR2: Dopamine receptor 1/2; GABA: γ-aminobutyric acid; TLR4: Toll-like receptor 4.