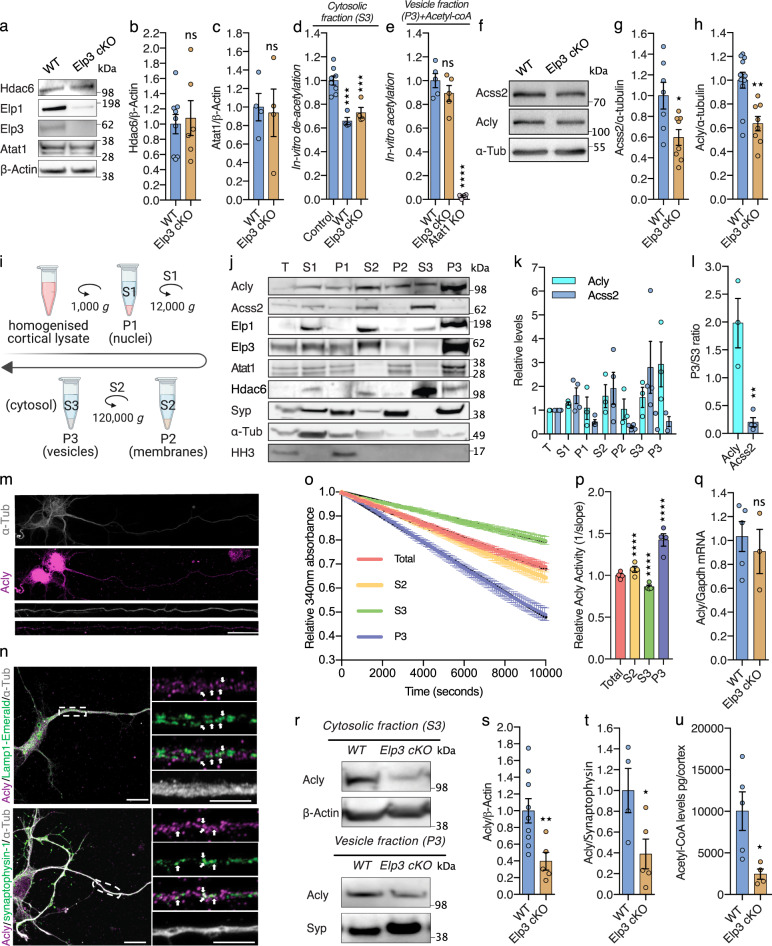
Fig. 2. Elongator deletion impairs tubulin acetylation and axonal transport via reduction of Acly-dependent acetyl-coA production.
a–c Western blotting to detect and quantify Hdac6, Elp1, Elp3, Atat1 (isoform #3 and #4)30, and ß-Actin in cortical extracts from newborn WT and Elp3 cKO mice. Histograms of proportion of Hdac6 b and Atat1 c expression to ß-Actin. d In vitro deacetylation assay of endogenously acetylated bovine brain tubulin incubated for 4 h with extracts (S3) of cortices isolated from WT, Elp3 cKO, or without tissue extract (control). e In vitro acetylation assay of nonacetylated MTs from HeLa cells incubated for 2 h with Acetyl-CoA and extracts (P3) of brain cortices isolated from WT, Elp3 cKO or Atat1 KO mice. f–h Immunoblotting to detect Acss2, Acly, and α-Tub cortical extracts from E14.5 WT and Elp3 cKO embryos and histograms of proportion of Acss2 (g) and Acly (h) expression to α-Tub. i Experimental pipeline for subcellular fractionation of P0 mouse cortical extracts: T, total; S1, post-nuclear; P1, nuclear; S2, cytosol and vesicles; P2, large membranes; S3, cytosol; and P3, vesicles. j–l Subcellular fractionation (T, S1, P1, S2, P2, S3, P3) of mouse brain cortex immunoblotted with specific antibodies to detect Acss2, Acly, Elp1, Elp3, Atat1 (isoform #3 and #4)30, Hdac6, synaptophysin (Syp), α-tubulin (α-Tub), and Histone H3 (HH3).k–l Histograms of proportion of Acss2 and Acly across subcellular fractions k or expressed as P3/S3 ratio l. Immunolabelings of E14.5 mice cortical neurons cultured for 5 DIV to detect Acly (purple) and α-tubulin (α-Tub, grey) m; Acly, Synaptophysin-1 (green) and α-Tub (n, top); Acly, Lamp-1-Emerald (green) and α-Tub (n, bottom). Scale bars are 20 µm (m), 10 µm (n). o–p Analysis of Acly activity by malate dehydrogenase coupled method performed in WT and Elp3 cKO brain cortex lysates. Histogram of relative Acly activity over assay (o) and of slopes (p) from the linear phase of the reaction. q qRT-PCR analysis of Acly mRNA in cortical brain extracts of Elp3 cKO or WT littermate mice.r–t Immunoblots and quantification of cytosolic (S3, s) and vesicular (P3, t) fractions of Acly protein from newborn WT and Elp3 cKO mice brain cortices. u LC-MS quantification of Acetyl-CoA levels in WT and Elp3 cKO P0 mice brain cortex lysates. Description of graphical summaries here within are histograms of means ± SEM. Significance was determined by: b, c, g, h, l, s, t two-sided t test, q, u two-sided Mann–Whitney test, d, e, p two-sided one-way analysis of variance (ANOVA), and o two-sided two-way ANOVA. Specifically, [(b) p = 0.8411, t = 0.2093, df = 6; (c) p = 0.7505, t = 0.3248, df = 13; (d) p < 0.0001, F = 21.30; (e) p < 0.0001, F = 85.29; (g) p = 0.0157, t = 0.2.776, df = 13; (h) p = 0.0013, t = 3.861, df = 17; (l) p = 0.0056, t = 4.638, df = 5; (o) p < 0.0001, FInteraction (294, 882) = 68.07; (p) p < 0.0001, F = 1352; (q) p = 0.5714, U = 5; (s) p = 0.0145, t = 2.855, df = 12; (t) p = 0.0428, t = 2.470, df = 7; (u) p = 0.0159, U = 0. In addition, the post hoc multiple comparisons, to analyze statistical difference` of each condition compared to control for (d, e, p) are Holm-Sidak test, and are *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Number of mice: (b) WT n = 9; Elp3 cKO n = 6; (c) WT n = 4; Elp3 cKO n = 4; (d) Control n = 8; WT n = 4; cKO n = 4; (e) WT n = 5; Elp3 cKO n = 5; Atat1 KO n = 4; (g) WT n = 7; Elp3 cKO n = 8; (h) WT n = 11; Elp3 cKO n = 8; (l) WT n = 3 (Acly); WT n = 4 (ACSS2); (o–p) WT n = 4 for 99 time points; (q) WT n = 5; Elp3 cKO n = 3; (s) WT n = 9; Elp3 cKO n = 5; (t) WT n = 4; Elp3 cKO n = 5; (u) WT n = 5; Elp3 cKO n = 4. e p = 0.031, t = 2.407, df = 13. Source data are provided with this paper.