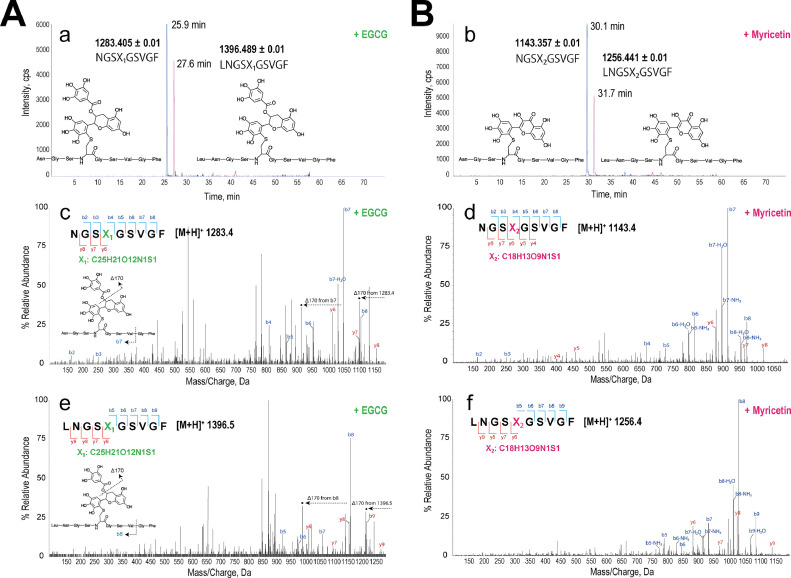
Fig. 3.
MS(/MS) analysis of covalently modified peptides at the active site. A; EGCG. B; Myricetin. After incubation, Mpro was digested with chymotrypsin. Extract ion chromatograms of the theoretical mass of EGCG- or myricetin-conjugated peptides with proposed chemical structures (a, b). X1 indicates EGCG-conjugated cysteine, and X2 indicates myricetin-conjugated cysteine. NGSX1/2GSVGF (1283.405 ± 0.01 (EGCG), 1143.357 ± 0.01 (myricetin)) and LNGSX1/2GSVGF (1396.489 ± 0.01 (EGCG), 1256.441 ± 0.01 (myricetin)) were the theoretical chymotrypsin-cleavable sequences (possible conjugated peptides) located at the active site. The MS/MS chromatograms generated from NGSX1/2GSVGF are shown in c (EGCG) and d (myricetin). Those of LNGSXGSVGF (1 missed cleavage) are shown in e (EGCG) and f (myricetin). Inset fragmentation schemes, c and e: EGCG-dependent characteristic fragments (Δ170) from the liberation of the galloyl moiety at the D ring in EGCG by collision-induced dissociation of EGCG-adducted peptides.