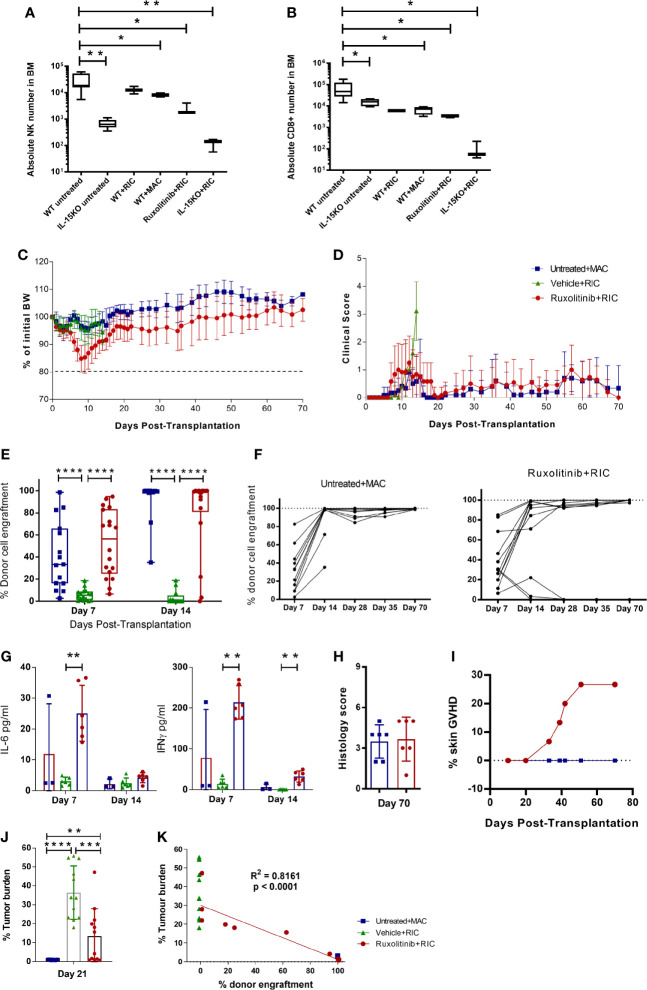
Figure 2.
Ruxolitinib treatment in combination with RIC mediates rapid and long-term donor cell engraftment, and permits GVT responses. Untreated WT and IL-15KO mice were compared to WT mice treated with RIC or MAC; or WT mice treated with ruxolitinib prior to RIC, or IL-15 KO mice treated with RIC. Mice were killed four days after receiving irradiation, and the absolute number of (A) NK cells (NKp46+CD49b+) and (B) CD8 (CD3+CD8+) T cells in BM were compared between different cohorts of mice (n=3-9/group). C57BL/6 WT mice were treated with ruxolitinib or vehicle for two days, and the following day treated with RIC and alloSCT. Another cohort of untreated WT mice was treated with MAC and alloSCT. Mice were monitored for (C) body weight and (D) clinical scores up to 70 days post alloSCT. (E, F) Donor cell engraftment (H2Kd+ cells) was monitored on days 7, 14, 28, 35 and 70 post-alloSCT in blood samples (n= 18, data combined from 3 independent experiments). (G) Plasma cytokine concentration of IFNγ and IL-6 was measured in blood samples collected on days 7 and 14 post-alloSCT. (H) Mice were killed 70 days post-alloSCT, and GVHD histology was conducted on gut tissue. (I) Incidence of development of skin GVHD in ruxolitinib+RIC mice compared to untreated+MAC alloSCT recipients (n=15). Mice were injected i.v. with MLL-AF9 tumour cells, and 8 days later were treated with ruxolitinib or vehicle for two days, and the following day treated with RIC and alloSCT. Another cohort of untreated WT mice was treated with MAC and alloSCT (n=12/treatment group, data combined from 2 independent experiments). (J) Mice were killed 21 days after alloSCT, and tumour burden was measured as a percentage of MLL-AF9+ cells in the BM. (K) Tumour burden was compared to donor cell engraftment between the untreated+MAC, vehicle+RIC and ruxolitinib+RIC cohorts 21 days after alloSCT. R2 value indicates the correlation between tumour burden and donor cell engraftment in ruxolitinib+RIC alloSCT recipients. Statistical analysis was performed using Ordinary One-way Anova Holm-Sidak’s multiple comparisons test (A, B), Mann-Whitney unpaired T test (E–J), and Pearson’s Correlation coefficient (K). *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001.