Highlights
-
•
Mood disorder (bipolar and unipolar) patients have aberrant functional connectivity.
-
•
We recovered functional networks at rest from mood disorder patients and controls.
-
•
Our method identifies transient brain activity changes and dynamic interactions.
-
•
Patients showed increased default-mode network (DMN) duration, but normal structure.
-
•
Depression modulated the duration of DMN, intrusive thoughts that of posterior DMN.
Keywords: Mood disorders, fMRI, Rumination, default-mode network (DMN), Total activation, Coactivation-patterns
Abbreviations: BD, bipolar disorder; BD-NOS, bipolar disorder non otherwise specified; MDD, Major Depressive Disorder; (p/a)DMN, (posterior/anterior) default-mode network; rs-fMRI, resting-state functional magnetic resonance imaging
Abstract
Spontaneous fluctuations in the blood oxygenation level dependent signal measured through resting-state functional magnetic resonance imaging have been corroborated to aggregate into multiple functional networks. Abnormal resting brain activity is observed in mood disorder patients, however with inconsistent results. How do such alterations relate to clinical symptoms; e.g., level of depression and rumination tendencies? Here we recovered spatially and temporally overlapping functional networks from 31 mood disorder patients and healthy controls during rest, by applying novel methods that identify transient changes in spontaneous brain activity. Our unique approach disentangles the dynamic engagement of resting-state networks unconstrained by the slow hemodynamic response. This time-varying characterization provides moment-to-moment information about functional networks in terms of their durations and dynamic coupling, and offers novel evidence for selective contributions to particular clinical symptoms. Patients showed increased duration of default-mode network (DMN), increased duration and occurrence of posterior DMN as well as insula- and amygdala-centered networks, but decreased occurrence of visual and anterior salience networks. Coupling between limbic (insula and amygdala) networks was also reduced. Depression level modulated DMN duration, whereas intrusive thoughts correlated with occurrence of insula and posterior DMN. Anatomical network organization was similar to controls. In sum, altered brain dynamics in mood disorder patients appear to mediate distinct clinical dimensions including increased self-processing, and decreased attention to external world.
1. Background
Mood disorders—both unipolar (Major Depressive Disorder, MDD) and Bipolar Disorder (BD) —are among the most prevalent and costly psychiatric disorders (James et al., 2018). They entail not only affective (e.g., sadness or anxiety), but also cognitive symptoms such as distractibility or rumination. The course of these disorders is difficult to predict or modify, and their underlying pathophysiology unclear. Several lines of evidence demonstrate aberrant brain activity in mood disorder patients (MDP) compared to healthy participants (HP) either during cognitive tasks or at rest, usually implicating multiple regions within large-scale networks (Han et al., 2018, Kang et al., 2016, Mulders et al., 2015, Sheline et al., 2010, Sheline et al., 2009, Wang et al., 2020). The nature and significance of these abnormalities and their relationships with clinical symptoms remain unresolved. However, in emerging views of precision psychiatry, it is of utmost importance to transition from simple associations between regional brain activity and broad phenotypes to more functional description of dynamically interacting and overlapping brain systems linked to particular clinical features (Silbersweig and Loscalzo, 2017). Here we investigate the dynamics of large-scale brain activity patterns to better characterize the functional changes and pathophysiology of mood disorders, with the ultimate aim to look for imaging-based biomarkers that may facilitate clinical assessment and intervention.
Resting-state functional magnetic resonance imaging (rs-fMRI) allows for non-invasive monitoring of brain activity without any specific task demand. Fluctuations of blood-oxygenated-level-dependent (BOLD) signals acquired during rest have been found to aggregate multiple regions with coherent activity, known as resting-state networks (RSNs) (Fox and Raichle, 2007). RSNs include the characteristic ‘task-negative’ or default mode network (DMN), as well as others that are reminiscent of task-positive networks, such as attention and sensory systems (Smith et al., 2009). These networks reflect large-scale functional organization and connectivity of the brain (Goldman et al., 2002, He et al., 2008, Mantini et al., 2007, Preti et al., 2016).
A growing body of studies point to altered activity of RSNs in MDP relative to HP. In MDD, several studies highlighted decreased cortico-limbic connectivity (Anand et al., 2005, Tang et al., 2013, Ye et al., 2016), whereas others reported increased connectivity of DMN with limbic and salience networks (Greicius et al., 2007, Sheline et al., 2010, Sheline et al., 2009). Systematic reviews (Kaiser et al., 2015a, Kaiser et al., 2015b, Mulders et al., 2015, Zhou et al., 2020) and metanalysis (Ma et al., 2019) converge to suggest that the anterior DMN (aDMN) shows hyperconnectivity, both intrinsically and with salience-related areas (i.e., insula and amygdala), while the posterior DMN (pDMN) shows hypoconnectivity with cognitive control areas. Moreover, anterior and posterior DMN tend to be more dissociated relative to HP (Mulders et al., 2015). However, another meta-analysis of rs-fMRI in MDD found that connectivity changes often affect brain regions that do not match with classical resting-state networks, and therefore suggested that these findings must reflect altered interactions between networks rather than only anomalies within networks (Sundermann et al., 2014). Other meta-analyses using whole-brain data with Activation Likelihood Estimation (ALE) method (applied to rest as well as emotional processing and cognitive tasks) found increased correlated activity in subcortical limbic areas such as amygdala, striatum, and thalamus (Palmer et al., 2014), as well as in the ventromedial prefrontal cortex (vmPFC), pregenual anterior cingulate cortex (pgACC), and parahippocampal regions (Kühn and Gallinat, 2013). Similarly, a metanalytic approach, based on peak coordinates from whole-brain resting-state functional data from 313 medication-naive first-episode MDD patients, reported major differences in the amygdala and parahippocampal gyrus compared to healthy controls (Ma et al., 2019).
Interestingly, activity in pgACC, parahippocampal gyrus, and DMN has been related to increased self-referential processing in unipolar patients (Grimm et al., 2009, Hamilton et al., 2011, Sheline et al., 2009), particularly with spontaneous and self-related negative thoughts, i.e., ruminations (Berman et al., 2011, Hamilton et al., 2011), as confirmed by a recent metanalysis as well (Zhou et al., 2020). Increased recruitment of DMN is therefore commonly considered as the biological basis for rumination and, consequently, causing interference on cognitive control networks (Andrews-Hanna et al., 2014, Marchetti et al., 2012).
However, the view that DMN is a task-negative network (passive) and globally anti-correlated with task-positive network is too simplistic (Koshino et al., 2014, Leech et al., 2011). Moreover, ruminations may involve differential activity across several brain areas (Piguet et al., 2014). Hence, the relationship between DMN connectivity, depression, and self-related thoughts such as rumination remains poorly understood. A few studies correlated changes in brain connectivity at rest with clinical variables and pointed to direct links between DMN or dorsomedial PFC (dmPFC) connectivity with mood scores on Hamilton Depression Rating Scale (Chen et al., 2021, Sheline et al., 2010) or the Ruminative Response Scale (Hamilton et al., 2011). In any case, impaired thought control and content remain a major clinical and dimensional variable of MDD, intimately associated with how the brain spontaneously functions at rest.
Furthermore, partly similar but also discordant observations have been reported for BD patients, consistent with a common pathophysiological model of mood disorders (Price and Drevets, 2012). Connectivity patterns of prefrontal areas (medial, ventral or dorsolateral) with meso-limbic areas such as the amygdala, thalamus, insula, or striatum were found to be either increased or decreased across different studies (for a review, see (Vargas et al., 2013), for a comparison between remitted and acute states (Wang et al., 2020), and for studies during remission (Syan et al., 2018)). Decreased connectivity between the posterior cingulate cortex (PCC) and striatum (Teng et al., 2014) or limbic regions (Liu et al., 2019) was also described in BD. However, despite the increasing body of evidence regarding resting state activity in BD, the complexity of the disease across variables episodes and clinical profiles, as well as the heterogeneity in the analysis methods, has prevented a definitive understanding of impairment in neural networks (Syan et al., 2018, Vargas et al., 2013).
Therefore, as for MDD, a better characterization of connectivity changes in BD is needed to better understand functional interactions both between and within brain networks (Khadka et al., 2013, Meda et al., 2014). Moreover, the specificity of findings in MDD and BD has to be clarified. Both differences and similarities between unipolar and bipolar patients were described (Anand et al., 2009, Chen et al., 2020, Fateh et al., 2020, Han et al., 2020, Liu et al., 2013, Liu et al., 2015, Luo et al., 2021, Nakamura et al., 2020, Yu et al., 2020, Zeng et al., 2020). For instance, recent work found decreased FC between limbic regions and the DMN in both BD and MDD patients (n = 55) as compared to HP (n = 24) (Liu et al., 2019). Interestingly, both BD (n = 41) and MDD (n = 61) patients presented decreased network switching rate in the DMN, while MDD patients had lower switching rate in salience network and striatum compared with BD and HP in a recent study on dynamic functional connectivity (Han et al., 2020). Finally, a recent study with seed-based functional connectivity analysis identified lower connectivity at prefronto-thalamo-cerebellar and sensorimotor-thalamic level in BD (n = 38) and MDD (n = 42) patients, as well as between the thalamus and the salience network in MDD patients only, but there was no correlation with clinical variables in any group (Zeng et al., 2020). In our study, we therefore chose to address these issues by focusing on clinical dimensions of mood disorder beyond dichotomous diagnostic categories, focusing on negative thoughts, anxiety, and intrusive thoughts.
New approaches to rs-fMRI analysis have shown that spontaneous brain dynamics can successfully be tracked to study moment-to-moment reorganization within and between networks, opening new ways to elucidate the pathophysiology of psychiatric disorders (Braun et al., 2018, Karahanoğlu and Van De Ville, 2017, Pillai and Jirsa, 2017, Preti et al., 2016). Several studies in HP (Allen et al., 2014, Chang and Glover, 2010, Hutchison et al., 2013, Liu and Duyn, 2013, Schaefer et al., 2014, Smith et al., 2012) and disease (Damaraju et al., 2014, Leonardi et al., 2013) examined the dynamic behavior of main network hubs with sliding-window correlations (Damaraju et al., 2014, Kaiser et al., 2016), revealing that different sub-components of RSNs may take part in different processes over time (Yeo et al., 2013, Yeo et al., 2011). Thus, fluctuations of DMN sub-components (Andrews-Hanna et al., 2010, Leech et al., 2011, Margulies et al., 2009) and their dynamic interactions provide important information about the intrinsic coordination and integrity of RSNs (Andrews-Hanna et al., 2014, Schaefer et al., 2014), which might be compromised in various psychiatric disorders. Another recent example of sliding-window approach is a multicenter study that found significantly lower amplitude of low-frequency fluctuation (ALFF) and fractional ALFF in the precuneus and posterior cingulate cortex (PCC) of 848 MDD patients compared to 794 HP (Yan et al., 2019).
In the current study, we sought to identify the building blocks of ongoing brain network activity using a novel method that allows for both spatial and temporal overlap. Specifically, this method captures transient activations through spatio-temporal fMRI deconvolution, and then defines innovation-driven co-activation patterns (iCAPs) containing all brain voxels with the same transient behavior (Karahanoğlu et al., 2013, Karahanoğlu and Ville, 2015). This approach conceptually accords with the hypothesis of discrete transitions between brain metastates (Vidaurre et al., 2017), although states in our framework are not mutually exclusive; i.e., they can be temporally overlapping. Compared with traditional, “static” connectivity analyses, which neglect time-varying information, dynamic fMRI methodologies capture fluctuations in neural activity to characterize ongoing changes in resting-state networks that overlap both spatially and temporally. Compared with ALFF or sliding window approaches, iCAP can thus detect instantaneous transients of activity of areas clustered together based on their activation pattern. This counteracts the effect of hemodynamic blurring, and can thus dissect temporally overlapping activity patterns, by determining consistent spatial patterns at the onset of de-activation or activation (Karahanoğlu and Ville, 2015).
Here, we extracted iCAPs from resting state fMRI in a group of mixed mood disorder patients in order to determine chages in both the temporal dynamics and spatial organization of spontaneous brain activity, relative to age-matched HP. Our transdiagnostic, dimensional approach is in line with current research models in psychiatry advising to target symptoms common to different pathologies (Cuthbert, 2020, Insel et al., 2010), rather than historically defined diagnoses. Accordingly, it is increasingly more frequent to target circuits across different diagnostic categories and relate them to clinical symptoms, rather than rely on fixed, historically-defined nosologic conditions. This approach may even increase our ability to uncover crucial alterations in mood disorders beyond specific diagnostic categories and thus help refine the current, historically defined divisions between these categories (Biswal, 2021). Examples of this are numerous (Gong et al., 2020, Luo et al., 2021). For instance a recent metanalysis of whole- brain rs-fMRI studies on ALFF in MDD and BD patients found aberrant regional intrinsic cerebral activity in common brain areas (i.e. mPFC, insula, and cerebellum) in both disorders (Gong et al., 2020). Another metanalysis found similar alterations in dynamic ALFF, calculated with a sliding-window approach, across the limbic system and primary visual area, in 901 BD, MDD, and schizophrenia patients compared to 384 healthy controls (Zhang et al., 2021). Our analyses focused on the occurrences, durations, and interactions of functional networks over time. We then examined the relationship between such changes in brain dynamics and particular clinical features. In line with dimensional models of MDD (Langenecker et al., 2014), we concentrated on negative mood, anxiety, and intrusive thoughts symptoms. We expected to observe longer duration of DMN or its subcomponents in patients, independently of their mood state, in accordance with perseverative thinking and reduced mental flexibility commonly reported in MDP. We also expected changes in cortico-subcortical limbic networks, in line with affective disturbances in patients. Critically, we predicted selective correlations of the duration and/or occurrence of particular iCAPs with these distinct clinical dimensions.
2. Methods and materials
2.1. Participants inclusion and characterization
Patients were recruited in the department of adult psychiatry at the Geneva University Hospital, as well as through advertising on classified advertisements websites. Healthy subjects were also selected from a local database or through web advertising. All subjects gave informed written consent before inclusion in the study that was approved by the ethical committee of the Geneva University Hospital. Inclusion criteria for patients were: a diagnostic of MDD or BD, age between 18 and 56, under stable medication for four weeks, and no contraindication for MRI. Exclusion criteria for healthy subjects were: past or present history of neurological or psychiatric problem, use of medication, or contraindication for MRI. The Mini International Neuropsychiatric Interview (MINI, Sheehan et al., 1998) and the Structured Clinical Interview for the DSM-IV, Mood Disorders section, (SCID, First et al., 2002) were administered to patients and healthy subjects for evaluation of current axis-I and axis-II diagnostic (during a separate visit for the patients). In total, 39 patients were initially recruited, however, 2 fell asleep in the MRI, 1 was claustrophobic, 2 were consuming cannabis on a daily basis, and 2 had a wrong diagnosis (ADHD-schizoaffective), and resting state data from one patient were lost for technical reason, leaving 31 data sets.
Our final sample therefore included 31 mood disorder patients (9 MDD and 22 BD) and 32 healthy participants (HP) matched for age, gender, hand laterality, and level of education (see Table 1). All underwent MRI scanning and clinical assessment. Clinical symptoms were measured with the Montgomery-Asberg Depression Rating Scale (MADRS; (Montgomery and Asberg, 1979; French version: Pellet et al., 1980), and Hamilton anxiety scale (Hamilton, 1959; French version: Pichot et al., 1981). For the Ruminative Response Scale (Nolen-Hoeksema and Morrow, 1991, Piguet et al., 2014), we chose only the brooding subscale, which represents the maladaptive component of ruminative response style (Treynor et al., 2003). We also asked participants to fill the White Bear Suppression Inventory (WBSI; (Schmidt et al., 2009), that measures the level of intrusive thoughts, and the Thought Control Ability Questionnaire (TCAQ; (Gay et al., 2008), in order to assess difficulties to control intrusive self-related thoughts as often seen in mood disorders (Ghaznavi and Deckersbach, 2012, Joormann et al., 2011). Patients were treated with different psychotropic drugs, sometimes more than one (anti-psychotic:N = 10, anti-depressant N = 15, mood stabilizer N = 11, benzodiazepine N = 5).
Table 1.
Demographic and clinical characteristics. BD-NOS: Bipolar disorder not otherwise specified.
| *p < .05 | Patients | Healthy participants | |
|---|---|---|---|
| Characteristics (min – max) | M (SD) | M (SD) | |
| N (males) | 27 (11) | 27 (11) | |
| Age | 39.7 (8.95) | 40 (8.6) | |
| Level of education (yrs) | 13.1 (3.1) | 13.96 (3.2) | |
| Laterality (not right-handed) | 20 (7) | 21 (6) | |
| MADRS (0–33) | 13.5 (8.9) | 2 (1.8) * | |
| Young (0–11) | 2.3 (2.9) | 0.5 (0.9) * | |
| Hamilton Anxiety (0–36) | 12.7 (7.5) | 3.2 (2.5) * | |
| RRS (22–82) | 55.4 (11.5) | 34.9 (10.4) * | |
| Brooding (5–20) | 12.4 (3.2) | 8.2 (2.5) * | |
| Mean age of onset | 24.9 (12.2) | ||
| Mean duration of disease | 14.8 (9.6) | ||
| Number of episodes: | |||
| 1–4 episodes | 8 | ||
| 5–10 episodes | 10 | ||
| >10 episodes | 9 | ||
| Diagnostic | Patients (N) |
|---|---|
| MDD | 7 |
| BD-I | 6 |
| BD-II | 11 |
| BD-NOS | 3 |
| Comorbidities | |
| Anxiety Dis | 13 |
| Borderline | 7 |
| ADHD | 2 |
| Episode | |
| depressive | 10 |
| euthymic | 10 |
| hypomanic | 2 |
| << mixed >> | 5 |
2.2. Data acquisition
Imaging data were acquired at the Brain and Behavior Laboratory (BBL) of UNIGE, using a Tim TRIO 3 T whole-body MR unit (Siemens, Germany) equipped with a 32 channels head coil. Functional time series were collected from 36 transverse slices covering the whole brain with a spatial resolution of 3.2x3.2x3.2 mm, using a 2D gradient-echo echo-planar (EPI) sequence (acquisition matrix = 64x64, FOV = 205 mm, TR/TE/FA = 2100 ms/30 ms/90°, 250 volumes). The total acquisition took around 8.5 mins. Furthermore, a T1-weighted volume for anatomical reference was collected with high-resolution three-dimensional T1-weighted MP-RAGE sequence with 0.9x0.9x0.9 mm spatial resolution (acquisition matrix = 256x256x192, FOV: 230 mm, TR/TE/FA/TI = 1900 ms/2.32 ms/9°/900 ms).
2.3. Data processing
The functional volumes were realigned with respect to the mean volume and smoothed (FWHM = 3 mm). The T1-weighted structural volumes were co-registered to the mean functional volume. The anatomical automatic labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) was mapped onto each subject’s coregistered anatomical image and further downsampled to match the functional volumes. The voxel time series were detrended for low frequency drifts (cut-off = 0.008 Hz). We computed maximum, mean and total measures as well as the frame-wise displacement (FDmm) and excluded five controls and four patients based on the following criteria: 1) fMRI data had visually detectable artifacts, or 2) maximum amount of motion was greater than 3 mm or 3°, or 3) number of frames with FD greater than 0.5 mm exceeded 25% of the total fMRI volumes. There were no group differences due to motion in the final sample, which consisted of 27 mood disorder patients and 27 controls (Table S2).
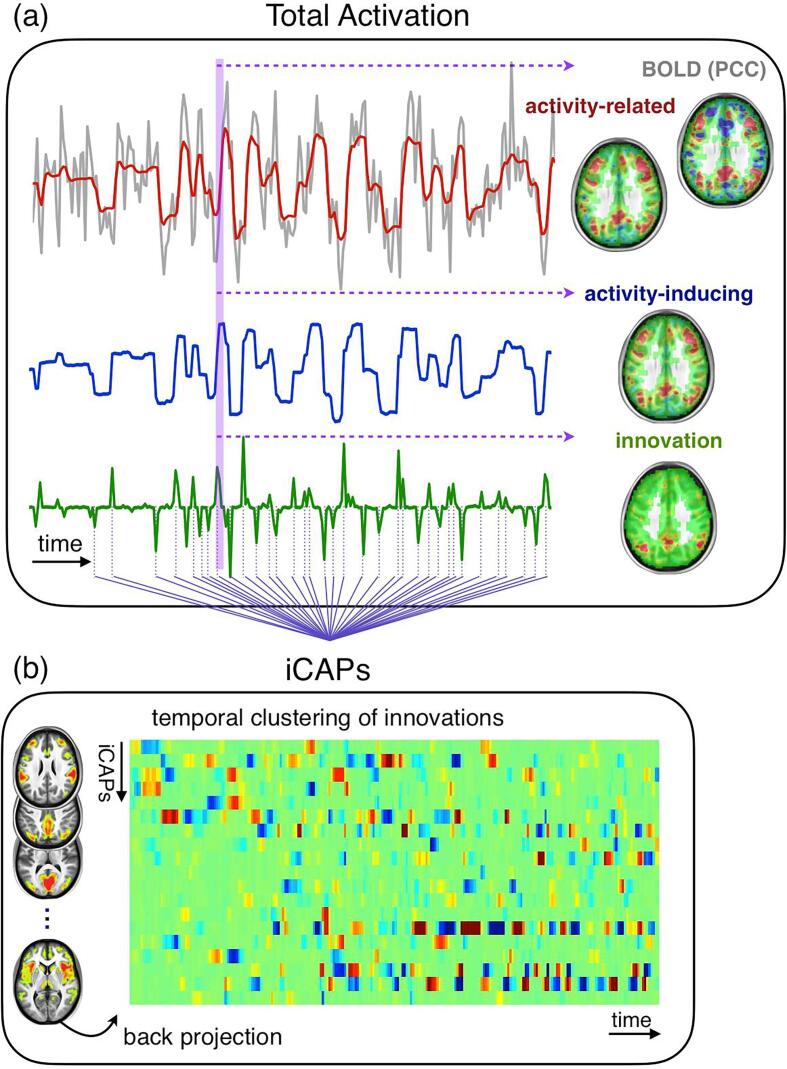
To assess dFC, we applied a novel state-of-the-art rs-fMRI data analysis tool, Total Activation (TA), which can detect spontaneous activity through transients as a result of voxel-wise deconvolution (Karahanoglu et al., 2011, Karahanoğlu et al., 2013). TA provides three types of information: (1) activity-related signals that are denoised fMRI signals; (2) sustained, or block-type, activity-inducing signals that are deconvolved signals; (3) innovation signals that are the derivative of the activity-inducing signals and encode transient brain activity episodes as spikes. Fig. 1 (a) illustrates the TA regularization scheme for a selected voxel time-series in posterior cingulate cortex (PCC). Specifically, the activity-related signal (first row, in red) opts for minimum error with respect to acquired BOLD signal (first row, in grey), while taking into account the hemodynamic response. The activity-inducing signal (second row, in blue) shows the block-like activation time-course when the hemodynamic component is removed. Lastly, the innovation signal (third row, in green) represents the transients encoding activation episodes. The brain maps (right column) for each type of signal at the delineated time instance (in purple shade) show that TA regularized voxels are grouped in clean activation clusters, whereby transients carry information about brief moments of spontaneous regional activity during rest. The analysis toolbox that we used for the implementation of the pipeline is available at https://miplab.epfl.ch/index.php/software/total-activation.
Fig. 1.
Total Activation and iCAP frameworks. (a) TA, here illustrated for a selected voxel in PCC for one subject, reveals block-like activity inducing signals (in blue) by denoising (activity-related signal in red) and deconvolving the BOLD signal (gray). The innovation signals (in green) are the derivatives of activity-inducing signals, and indicate the onset and offset timing of the events. The activation maps on the right correspond to the activation maps of each type of signal at a selected time frame (in purple shadow). (b) iCAPs are recovered by temporal clustering of innovation signals, and reveals spatially overlapping activation patterns with common onset timing. Backprojection of the spatial maps onto the activity-inducing signal results in highly overlapping temporal dynamics of iCAPs.
2.4. Temporal characterization of iCAPs’ time courses
Similar to the backprojection step in independent component analysis, iCAPs’ time series were recovered by backprojecting each iCAP into subjects’ activity-inducing signals; i.e., block-type activity representations recovered by TA. We computed three measures representing the temporal characterization of iCAPs: 1) occurrence, 2) average duration, and 3) total duration. We tested for differences in these measures between the groups using t-test followed by FDR correction (p ≤ 0.05 two tailed). Since total duration is a by-product of average duration and occurrence, we present the results for total duration in the Supporting Information.
2.5. Selection of iCAPs
In order to select the relevant iCAPs, the concatenated innovation signals of all included subjects were clustered via k-means (using cosine distance metric). To ensure stability and optimum solution, the algorithm was run 50 times with random initialization, and the best solution with minimum cost was chosen as the final iCAPs representations. We opted for 20 components, representing the group-level iCAPs, guided by our previous studies (Karahanoğlu and Ville, 2016, Karahanoğlu et al., 2013).
2.6. Relationship between iCAPs’ time courses and clinical measures
We tested the correlations of iCAPs’ temporal measures with clinical scores (MADRS, HAMA, Brooding, TCAQ and WBSI) using non-parametric permutation testing. We generated 10 k permutations by shuffling the subjects’ clinical scores and used the maximum statistic both across iCAPs and clinical scores to correct for multiple comparisons (Nichols and Holmes, 2002).
2.7. Temporal interactions of iCAPs
In order to explore the dynamic interactions during resting state, we computed the amount of temporal overlap and coupling of specific two iCAPs configurations; i.e., the iCAPs whose durations showed significant group differences. We first found significantly overlapping iCAPs in each group separately and then tested for group differences with permutation testing. To this aim, each iCAPs’ time series were shuffled 10 k times, and total time and percentage of sign dependent overlap was computed between each of these two iCAPs configurations. Maximum statistic was used to correct for multiple comparisons. The amount of overlap was computed by counting the time instances where any of the two specific iCAPs temporally overlapped during entire scan. Then, for each two iCAPs configuration we computed the percentage of the coupling; i.e., the total time of {(+,+),(-,-)} interactions divided by the total time of overlap, and anti-coupling; i.e., the total time of {(+,-),(-,+)} interactions divided by the total time of overlap. This metric shows the distribution of sign dependent two iCAPs configurations.
3. Results
3.1. Participants
Patients and controls differed on several clinical measures. The mean, standard deviation, and results from independent samples t-tests for these measures are reported in Table 1.
3.2. Innovation-driven co-activation patterns (iCAPs)
As described in the Methods (Paragraph 2.3), the voxel-wise transient signals defined by TA were then submitted to temporal clustering analysis to obtain innovation-driven co-activation patterns (iCAPs; (Karahanoğlu and Ville, 2015). Fig. 1 (a) third row illustrates the transients included in the temporal clustering for one voxel time series for one subject (dotted lines). While we opted for 20 iCAPs in k-means algorithm, 19 stable iCAPs were finally retained; i.e., one iCAP, driven by a single subject, was excluded (see Fig. S1 for group-level iCAPs, and Table S1 for distribution of regions within each iCAP). The iCAPs were sorted (from 1 to 19) according to their average duration during the scanning session.
We found that iCAPs were reminiscent of several common task-related and cognitive networks typically observed in fMRI studies, including the auditory network (6), high and low-level visual areas (2, 8, 9, 13, and 17), motor system (16), attention network (15), central executive network (7), and language network (3). In addition, areas involved in emotion and memory processing, including the amygdala and hippocampus, but also ventromedial prefrontal cortex, fusiform, and middle temporal gyrus (MTG), were aggregated in iCAP 12. Another insula-centered network (11) also encompassed subcortical and ventral frontal regions (thalamus, hippocampus, putamen, ACC, and vmPFC). The iCAPs 1 and 18 encompassed regions associated with the salience network (with anterior focus in iCAP 1), mainly ACC, middle and inferior frontal gyri, anterior insula, supramarginal gyrus, and temporal parietal junction (TPJ).
Finally, five iCAPs (4, 5, 10, 14, and 19) partially coincided with the canonical DMN. Both iCAPs 4 and 10 represented the full DMN with large sectors of high activation in PCC, precuneus, angular gyrus, inferior parietal lobule (IPL), and mPFC. The iCAP 4 further extended into the supramarginal gyri, TPJ, and middle temporal gyrus, unlike iCAP 10; whereas iCAP 10 captured more activity in frontal eye fields (FEF) and parahippocampal cortex. Dissociations between anterior and posterior parts of the DMN were present in several of these iCAPs. The iCAP 5 captured the entire mPFC including ACC. Conversely, the iCAP 14 was more centered on posterior areas such as PCC, precuneus, angular gyrus, supramarginal gyrus, middle/superior temporal gyrus, inferior occipital gyrus, as well as the thalamus; whereas the iCAP 19 was dominated by precuneus and adjacent areas in superior parietal lobes, superior occipital lobes, and PCC (see Figs. S2–S6 for the whole spatial extent of PCC-dominant iCAPs). These findings point to dissociable patterns of activity within the canonical DMN.
A direct comparison of the spatial organization of each iCAP between patients and controls was performed with non-parametric permutation inference testing (PALM, (Winkler et al., 2014), but did not result in any group differences. Thus, the intrinsic anatomical organization of functional networks revealed by our TA analysis does not seem to be altered in patients.
3.3. Temporal characterization of iCAPs
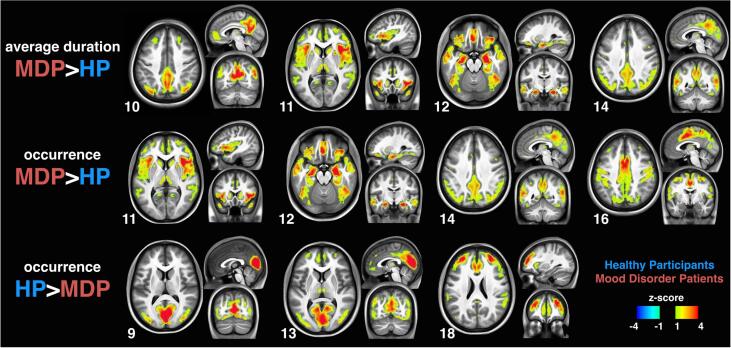
We next turned to the temporal dynamics of network activity in our two populations. The iCAPs’ time-series were recovered by back-projecting each iCAP map onto individual subjects’ activity-inducing signals (i.e., block-type activity representations identified by TA). Fig. 1 (b) depicts an example of iCAPs’ time-series from a single subject revealing dynamic fluctuations of multiple iCAPs over time. The time-series of the activity-inducing signals revealed vastly overlapping activity for different iCAPs. Accordingly, the total duration of all iCAPs (summed across every occurrence) was equally distributed among all subjects with 31.8 ± 2.6 min per subject; i.e., almost four times the 8.5 min scan duration. However, several temporal measures of iCAPs showed significant differences between the groups. Fig. 2 depicts those networks whose durations differed between groups in average duration and/or occurrence rate (pcorr-FDR ≤ 0.05 two tailed t-test FDR corrected; see Fig. S7 for comparison of all iCAPs and their total durations).
Fig. 2.
Group differences in iCAPs’ average duration and occurrence. The DMN (10), posterior DMN (14), insula (11) and amygdala (12) were significantly longer in patients. The iCAPs 11, 12, 14 and motor (16) had higher occurrence rate in patients, while iCAPs of visual areas (9&13) and anterior salience (18) had higher occurrence rate in controls.
The average durations of iCAPs corresponding to DMN (10; MDP:9.2 ± 1.7 s, HP:7.7 ± 1.8 s) and pDMN (14; MDP:8.5 ± 1.9 s, HP:7.1 ± 2.2 s), but also those of the insula-centered (11; MDP:8.8 ± 1.7 s, HP:7.6 ± 1.4 s) and amygdala-centered networks (12; MDP:8.5 ± 1.7 s, HP:7.4 ± 1.7 s) were longer in patients than controls. No iCAP had significantly longer average duration in controls than in patients.
In term of occurrence rate, three iCAPs were more frequently emerging in the time-series for patients than for controls: the insula-centered network (11; MDP:2 ± 0.1 per min, HP:1.4 ± 0.1 per min), pDMN (14; MDP:1.7 ± 0.1 per min, HP:0.7 ± 0.1 per min), and motor network (16; MDP:1.5 ± 0.1 per min, HP:1 ± 0.1 per min). Conversely, controls showed a higher occurrence rate of activity in primary visual areas (9; MDP:1.3 ± 0.1 per min, HP:1.7 ± 0.1 per min, and 13; MDP: 1.1 ± 0.1 per min, HP:1.6 ± 0.1 per min), and in the anterior salience network (18; MDP:0.7 ± 0.1 per min, HP:1.3 ± 0.1 per min).
3.4. Correlation of iCAPs’ dynamics with clinical scores
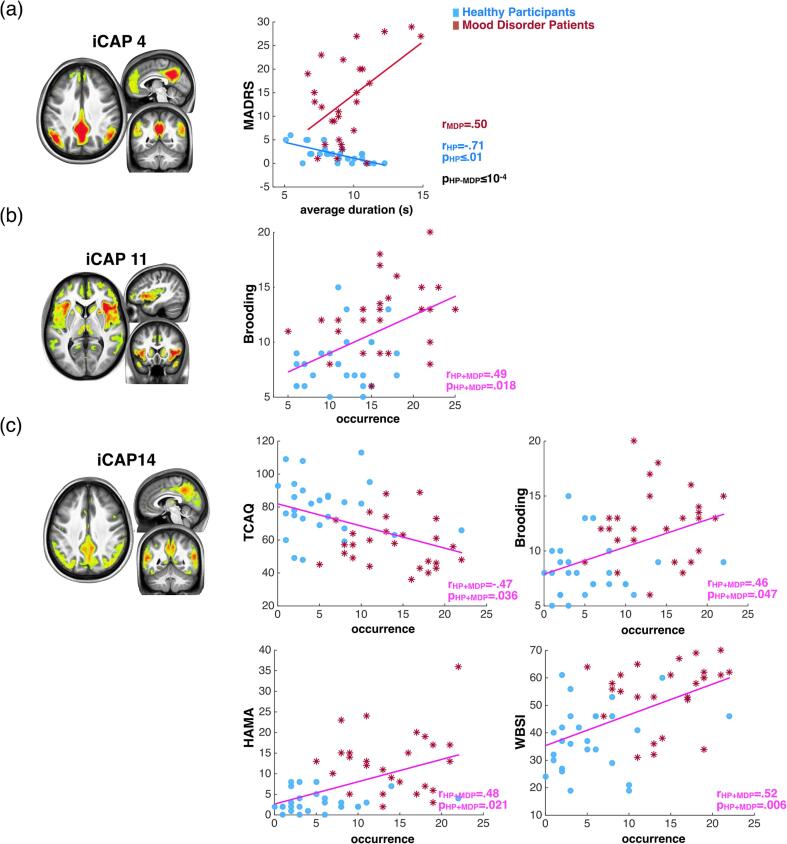
We tested whether our measures for the occurrence rate, average duration, and total duration of all 19 iCAPs were correlated with relevant clinical scores of mood disorder symptoms, both within and across groups to investigate group differences and trait level characteristics, respectively. We used non-parametric permutation testing with 10 k permutations, followed by multiple comparisons correction with maximum statistics across both iCAPs and clinical scores (Nichols and Holmes, 2002). Fig. 3 highlights those correlations between clinical scores and iCAPs’ average durations and occurrences with critical importance concerning hypotheses on the role of DMN.
Fig. 3.
Significant correlations between iCAPs durations and clinical scores. (a) There is significant group difference (in black) for correlation between MADRS score and average duration of DMN (4) which is negative and positive in controls (in blue) and in patients (in red), respectively. (b) The brooding score correlates with the occurrence of insula (11) in the combined group (in magenta). (c) The occurrence of pDMN (14) correlates with TCAQ, Brooding, HAMA and WBSI scores in the combined group (in magenta).
Again, underscoring the implication of DMN activity in mood disorders, we found that the MADRS score showed a positive trend correlation with the average duration of DMN (4) in patients, but a negative correlation in controls, leading to a significant group difference (pcorr < 10-4; Fig. 3(a)).
The number of occurrences of the insula-centered network (11) correlated positively with tendency to ruminate (Brooding pcorr = 0.018; Fig. 3(b)). The number of occurrences of pDMN (14) also correlated positively with trait rumination, as well as with intrusive thoughts (WBSI) and anxiety (HAMA), but it correlated negatively with thought control (TCAQ) (respectively, Brooding pcorr = 0.047; WBSI pcorr = 0.006; HAMA pcorr = 0.021; TCAQ pcorr = 0.036; see Fig. 3(c)).
Further exploratory results can be found in the Supplementary material. In particular, Fig. S8 illustrates significant correlations between clinical scores and the total duration of other iCAPs, as well as clinical score correlations with iCAPs’ durations that did not differ between groups.
3.5. iCAPs’ configurations of overlapping activity reveal disrupted resting-state dynamics
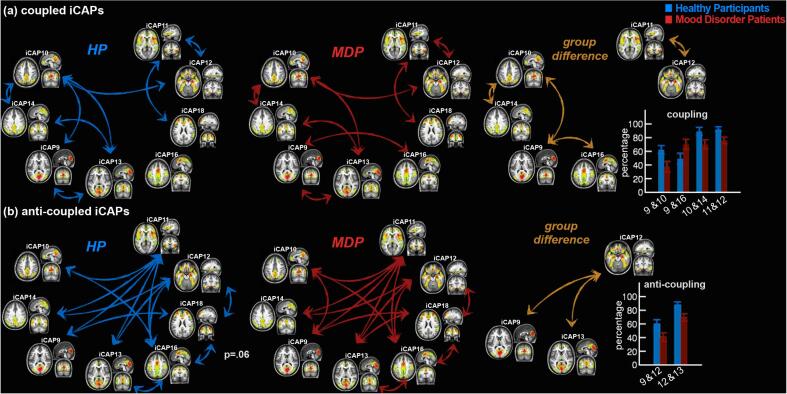
The temporal interactions between any two iCAPs whose durations showed group differences (Fig. 2) were investigated by measuring the total time of overlap, as well as the percentage of sign-dependent overlap (see Methods). The amount of total temporal overlap between insula—pDMN iCAPs (11&14) was significantly higher in patients than in controls (MDP: 10.9%, HP: 2.4% of the entire scan duration, p <10−4). While many iCAPs showed significant group differences in terms of duration (Fig. 2), only the iCAPs 11 and 14 overlap was more frequent in patients.
Further, when overlapping activity was considered in terms of polarity (percentage of sign-dependent overlap; i.e., {(+,+),(-,-)} for coupling and {(+,-),(-,+)} for anti-coupling between iCAPs), we observed globally similar patterns in both groups (Fig. 4). Specifically, coupled iCAPs consisting of DMN—pDMN (10&14), DMN—visual, pDMN—visual (10&13, 14&13), visual—visual (9&13), DMN—amygdala (10&12), insula—amygdala (11&12), and insula—anterior salience iCAPs (11&18) were significantly present in both controls (Fig. 4(a), left, in red) and patients (Fig. 4(a), middle, in blue). However, controls showed significantly more coupling between DMN—pDMN (10&14, pcorr = 0.016), insula—amygdala (11&12, pcorr = 0.034), as well as DMN—visual iCAPs (9&10, pcorr = 0.003), whereas patients showed more coupling between visual—motor iCAPs only (9&16, pcorr = 0.017) (Fig. 4(a), right, in orange). On the other hand, anti-coupled iCAPs were also common in both groups, involving many pairs of iCAPs (HP: Fig. 4(b), left, in red; MDP: Fig. 4(b), middle, in blue), particularly those implicating the insula (11), amygdala (12), pDMN (14), and motor areas (16). The only anti-coupled activity that differed between groups concerned the amygdala—visual iCAPs (9&12, pcorr = 0.028, 12&13, pcorr = 0.036), more evident in controls than in patients (Fig. 4(b), right, in orange).
Fig. 4.
Significant iCAPs couplings in controls and patients. (a) The significantly coupled iCAPs in controls (left, in blue), patients (middle, in red) and significant group differences (right, in orange) and the percentage of overlap (bar plots show the mean and the standard error). The coupling between amygdala and insula (12&11), DMN and pDMN (10&14), DMN and visual (10&9) are significantly higher in controls whereas visual and sensorimotor (9&16) are more coupled in patients. (b) The anti-coupled iCAPs in controls (left, blue), patients (middle, red) and significant group differences (right, orange). Amygdala and visual areas (12&9, 12&13) show higher anti-coupling in controls.
4. Discussion
Exploiting a novel data-driven approach tailored to disentangle transient activity of coordinated brain networks from spontaneous fMRI measurements, we unveil the resting-state dynamics in mood disorder patients (MDP) and matched healthy participants (HP), and relate changes observed in patients to particular clinical dimensions. While traditional fMRI methodologies are insensitive to such transient information, we were able to exploit ongoing fluctuations in neural activity to characterize the dynamics of resting-state networks that overlap both spatially and temporally. We first demonstrate a difference in the number of occurrences and duration of specific brain state configurations (i.e., innovation-driven co-activation patterns, iCAPs) between MDP and HP. Notably, the DMN was not only active for longer periods in patients, but its duration also correlated positively with level of depression in patients and negatively in controls (iCAP 10 and 4 respectively). Moreover, a posterior component of the DMN (pDMN, iCAP 14) also activated longer and more frequently in patients, but correlated specifically with tendency to ruminate, anxiety, intrusive thoughts, and poor thought control. Likewise, limbic networks centered on the insula (iCAP 11) and amygdala (iCAP 12) showed increased occurrences and durations, with the former associated with negative ruminations (brooding), whereas controls showed more frequent occurrences of visual (iCAPs 9 and 13) and anterior salience networks (iCAP 18, see Fig. 2). Importantly, we found no spatial differences in the anatomical configuration of any of these networks between the two groups. This result converges with others studies in MDP (Lois and Wessa, 2016, Wei et al., 2016, Zeng et al., 2020) and underlines the need for analyses sensitive to the temporal dynamics of activation patterns rather than simple spatial comparisons.
In addition, we could demonstrate changes in the coordination of activity across networks, with reduced coupling in the patients between the DMN—pDMN (10&14) and between the insula—amygdala iCAPs (11&12), as well as reduced anti-coupling between the amygdala—visual networks (12&9, 12&13). These results further highlight the utility of dynamical parameters characterizing functional brain networks to reveal pathological changes associated with psychiatric diseases, and open new perspectives to probe for changes linked to specific clinical features.
4.1. Increased presence of DMN and intrusive thoughts
Although data on resting connectivity in MDP are heterogeneous and still inconclusive, increased DMN activity is among the most reproduced finding, at least during unipolar (Kaiser et al., 2015a, Kaiser et al., 2015b, Mulders et al., 2015) or bipolar depression (He et al., 2016, Liu et al., 2015). Indeed, euthymic bipolar patients or those with a psychosis history may show an opposite pattern of DMN connectivity (Brady et al., 2017, Meda et al., 2014). In our study, increased duration of DMN (4) was associated with higher depression scores (MADRS). Although we considered all mood states together in a dimensional approach, our patients were mainly depressed or euthymic with residual depressive symptoms. Our results therefore appear consistent with previous studies, e.g., when comparing depressed to manic bipolar patients (Martino et al., 2016), or reporting correlations between increased DMN dominance at rest and depression scores on the RRS (Hamilton et al., 2011), or increased stability and connectivity of DMN in patients (Demirtaş et al., 2016). The evidence that the duration of DMN activity is longer and correlates with mood measures strongly argues in favor of a specific role of this network in the pathophysiology of depressive symptoms rather than a role in one diagnostic category. Nonetheless, it ought to be noted that reports of decreased stability of DMN in depressed patients also exists (Long et al., 2020).
However, in line with other studies exploring sub-networks of DMN (Andrews-Hanna et al., 2010, Manoliu et al., 2013, Meda et al., 2014) and a possible disconnection between its anterior and posterior sectors in MDP (Grimm et al., 2011, Lois and Wessa, 2016, Sambataro et al., 2014), our results also highlight a particular role for the pDMN/PCC region. This region exhibited not only increased duration and occurrence, but also selective correlation with tendency to ruminate as well as intrusive thoughts (WBSI), poor thoughts control (TCAQ), and anxiety. Increased PCC connectivity has previously been reported in MDD (Li et al., 2013, Manoliu et al., 2013, Sambataro et al., 2014, Sundermann et al., 2014), as well as in euthymic and non-euthymic patients with BD (Stoddard et al., 2016), and was even found to normalize after antidepressant medication (Li et al., 2013). The pDMN is implicated in consciousness and memory processing through its connections with the hippocampal formation (Andrews-Hanna et al., 2014, Cavanna and Trimble, 2006), as well as spontaneous thoughts and mind-wandering (Christoff et al., 2016).
Indeed, in MDD, previous studies reported that the severity of rumination may be predicted by the relative dominance of DMN, and more specifically pDMN versus task-positive networks at rest (Hamilton et al., 2013), as well as by increased connectivity between amygdala and PCC. Active rumination tasks also trigger activity in PCC along other medial and limbic structures in depressed patients (Burkhouse et al., 2016, Cooney et al., 2010). The PCC/pDMN activity might be implicated in “integrating self-relational information within a spatial–temporal context” (Hamilton et al., 2015), in keeping with the notion that PCC mediates self-attribution and “core self” representations (Davey et al., 2016). Self-referential tasks induce increased activity in posterior (and anterior) medial structures in both healthy and MDD individuals (Grimm et al., 2009, Lemogne et al., 2011). Accordingly, our results fit with the notion that intense self-related thoughts may partly emerge from PCC/pDMN activity, with more frequent and/or sustained recruitment in MDP.
4.2. Insula and amygdala-centered networks
Increased duration of a limbic amygdala-centered network (iCAPS 12 in particular) is consistent with the role of these regions in encoding affective salience and their enhanced activity in mood disorders, as observed by task-based fMRI studies in MDD (Palmer et al., 2014) or BD (Delvecchio et al., 2012, Houenou et al., 2011), and predicted by current pathophysiological models (Chase and Phillips, 2016, Price and Drevets, 2012). A recent graph-theory analysis also showed increased connectivity in an amygdala-based network, although this was more associated with hypomania than depression (Spielberg et al., 2016). Similarly, a recent paper applying regular CAPS rs-fMRI analysis in BD patients found significant differences in amygdala dynamic connectivity between different mood states (Rey et al., 2021). In our study, increased duration of the amygdala-centered network (12) extended to hippocampus/parahippocampal gyrus and medial/ventral prefrontal areas, as well as to the sgACC, all regions whose connectivity with amygdala is well established and frequently found abnormal in depression or euthymic BD patients (Rey et al., 2016).
Interestingly, increased connectivity of amygdala with PCC, another prominent region in our study, has been demonstrated in various mood states, including adolescent depression (Cullen et al., 2014, Peters et al., 2016) and bipolar patients (Rey et al., 2016). Increased connectivity between amygdala and precuneus was also observed (Singh et al., 2015). Here we did not find a direct association of these regions in one single iCAP including both amygdala and PCC/precuneus, however. While these findings accord with an involvement of these networks in disturbed integration between emotional and self-related processing in mood disorders, they also point to distinct dynamics and different roles in psychopathological processes. The occurrence rate of pDMN correlated with various measures of intrusive thoughts, whereas the occurrence of the insula-centered iCAP was associated with rumination only.
This observation adds to previous findings in the literature linking the DMN to rumination (Zhou et al., 2020). For example, functional connectivity between sgACC and insula was increased during active ruminative state compared to positive mood in healthy subjects (Milazzo et al., 2016), while more variable connectivity between mPFC and insula correlated with rumination in unmedicated MDD patients (Kaiser et al., 2016). Anterior insula activation might subtend a particular content of ruminative thoughts in relation to interoception and attention to internal bodily states (Keysers and Gazzola, 2007), potentially amplified in depression and anxiety (Paulus and Stein, 2010). Anterior insula is also implicated in encoding aversive signals across modalities and negative emotional arousal (Corradi-Dell’Acqua et al., 2016, Knutson et al., 2014). This may account for our finding of insula-based network activity associated with the brooding dimension of ruminations.
Very interestingly, while both networks were more present in patients, the temporal coupling of insula—amygdala iCAPs (11&12) was significantly lower relative to controls. Since connectivity between these regions might be associated with emotion regulation strategies (Denny et al., 2014), such reduced coupling might reflect maladaptive strategies associated with rumination. Alternatively, impaired synchronization between these two networks could be associated with emotion dysregulation in general. This would accord with another study in which borderline personality patients, known for emotional lability and anxiety, also showed lower connectivity between insula and amygdala as compared to controls (Koenigsberg et al., 2014). More generally, our results thus indicate that psychiatric disturbances may not only imply abnormal activity within specific brain networks, but also abnormal coordination between different networks. Further research is needed to better elucidate the functional nature and significance of such cross-talks between networks.
4.3. Increased interoception and decreased external focus in mood disorders patients
Finally, unlike MDP, our HP showed longer duration of prefrontal (18) and visual networks (9, 13). This pattern dovetails with current models (Phillips et al., 2008, Price and Drevets, 2012, Strakowski et al., 2012) proposing that a decrease in cognitive control subtended by prefrontal areas is associated with increased limbic activity in mood disorders patients. As discussed above, such increases in limbic-related activity might reflect enhanced responsiveness to affective salience partly mediated by amygdala activity (Ye et al., 2016), or heightened focus on self-reflective and interoceptive information mediated by insula activity (Adolfi et al., 2016). Additionally, patients may spend less time engaging attention toward visual processes (i.e., for direct sensory experience or mental imagery), while focusing more on internal affective and memory information (Piguet et al., 2016). Accordingly, visual areas were less coupled with DMN in our patients (10&9), while they were more anti-coupled with the amygdala network (12&9, 12&13). Taken together, these data suggest a desynchronization between external-based processes and more internally-based ones. These results reinforce the idea of increased internally-focused attention in mood disorder patients, with less attention to external cues and lower ability to switch away from negative self-related thoughts (Hamilton et al., 2013, Marchetti et al., 2012).
Other studies suggested that exaggerated self-related thoughts may result from an interference of negative material over cognitive control, with impaired interactions between corresponding brain regions (Kaiser et al., 2015a, Kaiser et al., 2015b). However, these studies did not directly assess the dynamics of specific networks, contrarily to our study. Importantly, our findings question the stability of these networks and putative impairments in switching between them. For example, insula activity has been associated with switching functions to recruit task-negative vs task-positive networks (Sridharan et al., 2008), and the more frequent occurrence of this network in our data may actually reflect more frequent switches between states in patients. The fact that the duration of these iCAPs was not necessarily related to their coupling argues for less stable network stability in patients.
Our unique approach can thus disentangle previous findings in mood disorders that relied upon conventional “static” functional connectivity, by assessing the dynamic engagement of functional networks characterized through time-resolved analysis. Notably, the patterns representing coherent transient activity during rest revealed multiple subcomponents of the DMN that are spatially and temporally overlapping, which concurs with recent dynamic accounts for spontaneous thought processes (Christoff et al., 2016). Moreover, the temporal properties (i.e., durations and couplings) of these recurring patterns provide novel evidence for selective contributions to particular clinical symptoms.
5. Conclusions and limitations
Leveraging recent advances in analysis of resting-state fMRI dynamics, we show that mood disorder patients present with an abnormal recruitment of DMN that correlates with their level of depression. An increased presence, in terms of duration and occurrence rate, of the posterior DMN component (centered on PCC) and limbic networks (centered on amygdala and insula) predicted intrusive thoughts, regardless of mood. In addition, the dynamic organization of coupling and anti-coupling of these networks was also altered in patients. As these changes in duration/occurrence of networks did not match their patterns of coupling/anti-coupling, our results might be interpreted as evidence of increased network instability among patients. Remarkably, changes in brain dynamics were found despite normal spatial network architecture.
Our study has some limitations, including first of all its small sample size, that warrants validation in larger samples. Secondly, clinical heterogeneity among patients could contribute to both the strength and the limitation of our work. On one hand, the variability of clinical features and treatments in our population might constitute confounding factors. On the other hand, we believe that this variability fruitfully reflects clinical reality as well as the variety of functional processes underlying mood disorders. Moreover, dimensional approaches such as ours allow for going beyond somewhat arbitrary, historically-defined boundaries between diagnostic categories (Cuthbert, 2020, Insel et al., 2010).
Another limitation is that our TA method does not take into account any variability of the hemodynamic model across subjects; like most fMRI analysis tools (Buxton et al., 1998). Future work might usefully include a derivation of region-specific hemodynamic response. Since the TA algorithm runs in both temporal and spatial domains iteratively, the total computation time heavily depends on the data dimension (i.e., it took 7–9 h of processing time per subject for 10 k-15 k voxel time series, using a Mac Pro server with 3.33 GHz 6-Core Intel Xeon Processor and 32 GB 1.33 GHz RAM). Refining processing steps will therefore benefit from future advances in computing power. Another limitation, shared by all clustering methods, is the choice of the number of clusters to define activated networks. Here, we opted for 20 clusters based on our previous reproducibility study (Karahanoğlu and Van De Ville, 2016), then ran k-means algorithm repetitively with random initializations for algorithmic reproducibility, and finally excluded any unstable cluster that was not well-represented in the whole group. While this ensures reliability and reproducibility, it may also suffer from insufficient sensitivity to more subtle signals. Finally, although our method allows for novel observations on network dynamics during resting state, it is purely data-driven and does not test specific models of mood disorder. In any case, our new results may fruitfully serve to refine and complement these models.
Altogether, despite the known caveats concerning reverse-inferences (Poldrack, 2006), our findings provide novel evidence supporting a model whereby mood disorders may be associated with higher internally focused-attention at the expense of more externally focused attention and difficulties with cognitive control, potentially leading to common clinical symptoms such as rumination, somatoform bodily complaints, and impaired cognitive flexibility, in addition to low mood and emotional dysregulation.
Funding
This work was supported in part by the Swiss National Science Foundation (grant #205321_163376) to D.V.D.V. and F.I.K; the NARSAD Independent Investigator Grant (#22174) provided by the Brain & Behavior Research Foundation (D.V.D.V.); C.P. was supported by a clinician-scientist grant from the Swiss National Center of Competence in Research, “Synapsy: the Synaptic Basis of Mental Diseases” [grant number. 51NF40-158776].
CRediT authorship contribution statement
Camille Piguet: Conceptualization, Investigation, Writing – original draft, Funding acquisition. Fikret Işık Karahanoğlu: Conceptualization, Methodology, Investigation, Writing – original draft. Luigi Francesco Saccaro: Writing – review & editing. Dimitri Van De Ville: Conceptualization, Methodology, Writing – review & editing, Funding acquisition, Resources, Supervision. Patrik Vuilleumier: Conceptualization, Writing – review & editing, Funding acquisition, Resources, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to thank the Brain and Behavior Laboratory (BBL) of Geneva University, where images were acquired.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102833.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adolfi F., Couto B., Richter F., Decety J., Lopez J., Sigman M., Manes F., Ibáñez A. Convergence of interoception, emotion, and social cognition: A twofold fMRI meta-analysis and lesion approach. Cortex. J. Devoted Study Nerv. Syst. Behav. 2016;88:124–142. doi: 10.1016/j.cortex.2016.12.019. [DOI] [PubMed] [Google Scholar]
- Allen E.A., Damaraju E., Plis S.M., Erhardt E.B., Eichele T., Calhoun V.D. Tracking whole-brain connectivity dynamics in the resting state. Cereb. Cortex N. Y. N. 2014;1991(24):663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A., Li Y., Wang Y., Lowe M.J., Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 2009;171(3):189–198. doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A., Li Y.u., Wang Y., Wu J., Gao S., Bukhari L., Mathews V.P., Kalnin A., Lowe M.J. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol. Psychiatry. 2005;57(10):1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M.G., Peltier S., Nee D.E., Kross E., Deldin P.J., Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6(5):548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B.K.K. Springer; Cham: 2021. Review of Resting-State Functional Connectivity Methods and Application in Clinical Populations. [Google Scholar]
- Brady R.O., Tandon N., Masters G.A., Margolis A., Cohen B.M., Keshavan M., Öngür D. Differential brain network activity across mood states in bipolar disorder. J. Affect. Disord. 2017;207:367–376. doi: 10.1016/j.jad.2016.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U., Schaefer A., Betzel R.F., Tost H., Meyer-Lindenberg A., Bassett D.S. From Maps to Multi-dimensional Network Mechanisms of Mental Disorders. Neuron. 2018;97(1):14–31. doi: 10.1016/j.neuron.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse K.L., Jacobs R.H., Peters A.T., Ajilore O., Watkins E.R., Langenecker S.A. Neural correlates of rumination in adolescents with remitted major depressive disorder and healthy controls. Cogn. Affect. Behav. Neurosci. 2016;17(2):394–405. doi: 10.3758/s13415-016-0486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R.B., Wong E.C., Frank L.R. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn. Reson. Med. 1998;39(6):855–864. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chang C., Glover G.H. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage. 2010;50(1):81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H.W., Phillips M.L. Elucidating neural network functional connectivity abnormalities in bipolar disorder: toward a harmonized methodological approach. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1(3):288–298. doi: 10.1016/j.bpsc.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Chen P., Gong J., Jia Y., Zhong S., Chen F., Wang J., Luo Z., Qi Z., Huang L., Wang Y. Shared and specific patterns of dynamic functional connectivity variability of striato-cortical circuitry in unmedicated bipolar and major depressive disorders. Psychol. Med. 2020;1–10 doi: 10.1017/S0033291720002378. [DOI] [PubMed] [Google Scholar]
- Chen V.-H., Chou Y.-S., Tsai Y.-H., Huang Y.-C., McIntyre R.S., Weng J.-C. Resting-State Functional Connectivity and Brain Network Abnormalities in Depressive Patients with Suicidal Ideation. Brain Topogr. 2021;34(2):234–244. doi: 10.1007/s10548-020-00817-x. [DOI] [PubMed] [Google Scholar]
- Christoff K., Irving Z.C., Fox K.C.R., Spreng R.N., Andrews-Hanna J.R. Mind-wandering as spontaneous thought: a dynamic framework. Nat. Rev. Neurosci. 2016;17(11):718–731. doi: 10.1038/nrn.2016.113. [DOI] [PubMed] [Google Scholar]
- Cooney R.E., Joormann J., Eugène F., Dennis E.L., Gotlib I.H. Neural correlates of rumination in depression. Cogn Affect Behav Neurosci. 2010;10(4):470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi-Dell’Acqua C., Tusche A., Vuilleumier P., Singer T. Cross-modal representations of first-hand and vicarious pain, disgust and fairness in insular and cingulate cortex. Nat. Commun. 2016;7:10904. doi: 10.1038/ncomms10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K.R., Westlund M.K., Klimes-Dougan B., Mueller B.A., Houri A., Eberly L.E., Lim K.O. Abnormal Amygdala Resting-State Functional Connectivity in Adolescent Depression. JAMA Psychiatry. 2014;71(10):1138. doi: 10.1001/jamapsychiatry.2014.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B.N. The role of RDoC in future classification of mental disorders. Dialogues Clin. Neurosci. 2020;22:81–85. doi: 10.31887/DCNS.2020.22.1/bcuthbert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E., Allen E.A., Belger A., Ford J.M., McEwen S., Mathalon D.H., Mueller B.A., Pearlson G.D., Potkin S.G., Preda A., Turner J.A., Vaidya J.G., van Erp T.G., Calhoun V.D. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage Clin. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey C.G., Pujol J., Harrison B.J. Mapping the self in the brain’s default mode network. NeuroImage. 2016;132:390–397. doi: 10.1016/j.neuroimage.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Delvecchio G., Fossati P., Boyer P., Brambilla P., Falkai P., Gruber O., Hietala J., Lawrie S.M., Martinot J.-L., McIntosh A.M., Meisenzahl E., Frangou S. Common and distinct neural correlates of emotional processing in Bipolar Disorder and Major Depressive Disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2012;22(2):100–113. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Demirtaş M., Tornador C., Falcón C., López‐Solà M., Hernández‐Ribas R., Pujol J., Menchón J.M., Ritter P., Cardoner N., Soriano‐Mas C., Deco G. Dynamic functional connectivity reveals altered variability in functional connectivity among patients with major depressive disorder. Hum. Brain Mapp. 2016;37(8):2918–2930. doi: 10.1002/hbm.v37.810.1002/hbm.23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny B.T., Fan J., Liu X., Guerreri S., Mayson S.J., Rimsky L., New A.S., Siever L.J., Koenigsberg H.W. Insula-amygdala functional connectivity is correlated with habituation to repeated negative images. Soc. Cogn. Affect. Neurosci. 2014;9:1660–1667. doi: 10.1093/scan/nst160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fateh A.A., Cui Q., Duan X., Yang Y., Chen Y., Li D., He Z., Chen H. Disrupted dynamic functional connectivity in right amygdalar subregions differentiates bipolar disorder from major depressive disorder. Psychiatry Res. Neuroimaging. 2020;304:111149. doi: 10.1016/j.pscychresns.2020.111149. [DOI] [PubMed] [Google Scholar]
- First Michael, Spitzer R.L., Gibbon M.L., Williams Janet. Research Version; Non-patient Edition: 2002. Structured clinical interview for DSM-IV-TR Axis I Disorders. [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gay P., d’Acremont M., Schmidt R.E., Van der Linden M. Validation of a French Adaptation of the Thought Control Ability Questionnaire. Eur. J. Psychol. Assess. 2008;24(2):101–107. doi: 10.1027/1015-5759.24.2.101. [DOI] [Google Scholar]
- Ghaznavi S., Deckersbach T. Rumination in bipolar disorder: evidence for an unquiet mind. Biol. Mood Anxiety Disord. 2012;2:2. doi: 10.1186/2045-5380-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R.I., Stern J.M., Engel J., Cohen M.S. Simultaneous EEG and fMRI of the alpha rhythm. NeuroReport. 2002;13(18):2487–2492. doi: 10.1097/00001756-200212200-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Wang J., Qiu S., Chen P., Luo Z., Wang J., Huang L., Wang Y. Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: voxel-based meta-analysis. Transl. Psychiatry. 2020;10:353. doi: 10.1038/s41398-020-01036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., Glover G.H., Solvason H.B., Kenna H., Reiss A.L., Schatzberg A.F. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S., Ernst J., Boesiger P., Schuepbach D., Boeker H., Northoff G. Reduced negative BOLD responses in the default-mode network and increased self-focus in depression. World J Biol Psychiatry. 2011;12(8):627–637. doi: 10.3109/15622975.2010.545145. [DOI] [PubMed] [Google Scholar]
- Grimm S., Ernst J., Boesiger P., Schuepbach D., Hell D., Boeker H., Northoff G. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Hum. Brain Mapp. 2009;30(8):2617–2627. doi: 10.1002/hbm.v30:810.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Chen M.C., Gotlib I.H. Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiol. Dis. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol. Psychiatry. 2015;78(4):224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M.M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Han K.-M., De Berardis D., Fornaro M., Kim Y.-K. Differentiating between bipolar and unipolar depression in functional and structural MRI studies. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018 doi: 10.1016/j.pnpbp.2018.03.022. [DOI] [PubMed] [Google Scholar]
- Han S., Cui Q., Wang X., Li L., Li D., He Z., Guo X., Fan Y.-S., Guo J., Sheng W., Lu F., Chen H. Resting state functional network switching rate is differently altered in bipolar disorder and major depressive disorder. Hum. Brain Mapp. 2020;41:3295–3304. doi: 10.1002/hbm.25017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J., Snyder A.Z., Zempel J.M., Smyth M.D., Raichle M.E. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Yu Q., Du Y., Vergara V., Victor T.A., Drevets W.C., Savitz J.B., Jiang T., Sui J., Calhoun V.D. Resting-state functional network connectivity in prefrontal regions differs between unmedicated patients with bipolar and major depressive disorders. J. Affect. Disord. 2016;190:483–493. doi: 10.1016/j.jad.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houenou J., Frommberger J., Carde S., Glasbrenner M., Diener C., Leboyer M., Wessa M. Neuroimaging-based markers of bipolar disorder: evidence from two meta-analyses. J. Affect. Disord. 2011;132:344–355. doi: 10.1016/j.jad.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Hutchison R.M., Womelsdorf T., Allen E.A., Bandettini P.A., Calhoun V.D., Corbetta M., Della Penna S., Duyn J.H., Glover G.H., Gonzalez-Castillo J., Handwerker D.A., Keilholz S., Kiviniemi V., Leopold D.A., de Pasquale F., Sporns O., Walter M., Chang C. Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K., Sanislow C., Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., Abdollahpour I., Abdulkader R.S., Abebe Z., Abera S.F., Abil O.Z., Abraha H.N., Abu-Raddad L.J., Abu-Rmeileh N.M.E., Accrombessi M.M.K., Acharya D., Acharya P., Ackerman I.N., Adamu A.A., Adebayo O.M., Adekanmbi V., Adetokunboh O.O., Adib M.G., Adsuar J.C., Afanvi K.A., Afarideh M., Afshin A., Agarwal G., Agesa K.M., Aggarwal R., Aghayan S.A., Agrawal S., Ahmadi A., Ahmadi M., Ahmadieh H., Ahmed M.B., Aichour A.N., Aichour I., Aichour M.T.E., Akinyemiju T., Akseer N., Al-Aly Z., Al-Eyadhy A., Al-Mekhlafi H.M., Al-Raddadi R.M., Alahdab F., Alam K., Alam T., Alashi A., Alavian S.M., Alene K.A., Alijanzadeh M., Alizadeh-Navaei R., Aljunid S.M., Alkerwi A., Alla F., Allebeck P., Alouani M.M.L., Altirkawi K., Alvis-Guzman N., Amare A.T., Aminde L.N., Ammar W., Amoako Y.A., Anber N.H., Andrei C.L., Androudi S., Animut M.D., Anjomshoa M., Ansha M.G., Antonio C.A.T., Anwari P., Arabloo J., Arauz A., Aremu O., Ariani F., Armoon B., Ärnlöv J., Arora A., Artaman A., Aryal K.K., Asayesh H., Asghar R.J., Ataro Z., Atre S.R., Ausloos M., Avila-Burgos L., Avokpaho E.F.G.A., Awasthi A., Ayala Quintanilla B.P., Ayer R., Azzopardi P.S., Babazadeh A., Badali H., Badawi A., Bali A.G., Ballesteros K.E., Ballew S.H., Banach M., Banoub J.A.M., Banstola A., Barac A., Barboza M.A., Barker-Collo S.L., Bärnighausen T.W., Barrero L.H., Baune B.T., Bazargan-Hejazi S., Bedi N., Beghi E., Behzadifar M., Behzadifar M., Béjot Y., Belachew A.B., Belay Y.A., Bell M.L., Bello A.K., Bensenor I.M., Bernabe E., Bernstein R.S., Beuran M., Beyranvand T., Bhala N., Bhattarai S., Bhaumik S., Bhutta Z.A., Biadgo B., Bijani A., Bikbov B., Bilano V., Bililign N., Bin Sayeed M.S., Bisanzio D., Blacker B.F., Blyth F.M., Bou-Orm I.R., Boufous S., Bourne R., Brady O.J., Brainin M., Brant L.C., Brazinova A., Breitborde N.J.K., Brenner H., Briant P.S., Briggs A.M., Briko A.N., Britton G., Brugha T., Buchbinder R., Busse R., Butt Z.A., Cahuana-Hurtado L., Cano J., Cárdenas R., Carrero J.J., Carter A., Carvalho F., Castañeda-Orjuela C.A., Castillo Rivas J., Castro F., Catalá-López F., Cercy K.M., Cerin E., Chaiah Y., Chang A.R., Chang H.-Y., Chang J.-C., Charlson F.J., Chattopadhyay A., Chattu V.K., Chaturvedi P., Chiang P.-P.-C., Chin K.L., Chitheer A., Choi J.-Y.-J., Chowdhury R., Christensen H., Christopher D.J., Cicuttini F.M., Ciobanu L.G., Cirillo M., Claro R.M., Collado-Mateo D., Cooper C., Coresh J., Cortesi P.A., Cortinovis M., Costa M., Cousin E., Criqui M.H., Cromwell E.A., Cross M., Crump J.A., Dadi A.F., Dandona L., Dandona R., Dargan P.I., Daryani A., Das Gupta R., Das Neves J., Dasa T.T., Davey G., Davis A.C., Davitoiu D.V., De Courten B., De La Hoz F.P., De Leo D., De Neve J.-W., Degefa M.G., Degenhardt L., Deiparine S., Dellavalle R.P., Demoz G.T., Deribe K., Dervenis N., Des Jarlais D.C., Dessie G.A., Dey S., Dharmaratne S.D., Dinberu M.T., Dirac M.A., Djalalinia S., Doan L., Dokova K., Doku D.T., Dorsey E.R., Doyle K.E., Driscoll T.R., Dubey M., Dubljanin E., Duken E.E., Duncan B.B., Duraes A.R., Ebrahimi H., Ebrahimpour S., Echko M.M., Edvardsson D., Effiong A., Ehrlich J.R., El Bcheraoui C., El Sayed Zaki M., El-Khatib Z., Elkout H., Elyazar I.R.F., Enayati A., Endries A.Y., Er B., Erskine H.E., Eshrati B., Eskandarieh S., Esteghamati A., Esteghamati S., Fakhim H., Fallah Omrani V., Faramarzi M., Fareed M., Farhadi F., Farid T.A., Farinha C.S.E.sá, Farioli A., Faro A., Farvid M.S., Farzadfar F., Feigin V.L., Fentahun N., Fereshtehnejad S.-M., Fernandes E., Fernandes J.C., Ferrari A.J., Feyissa G.T., Filip I., Fischer F., Fitzmaurice C., Foigt N.A., Foreman K.J., Fox J., Frank T.D., Fukumoto T., Fullman N., Fürst T., Furtado J.M., Futran N.D., Gall S., Ganji M., Gankpe F.G., Garcia-Basteiro A.L., Gardner W.M., Gebre A.K., Gebremedhin A.T., Gebremichael T.G., Gelano T.F., Geleijnse J.M., Genova-Maleras R., Geramo Y.C.D., Gething P.W., Gezae K.E., Ghadiri K., Ghasemi Falavarjani K., Ghasemi-Kasman M., Ghimire M., Ghosh R., Ghoshal A.G., Giampaoli S., Gill P.S., Gill T.K., Ginawi I.A., Giussani G., Gnedovskaya E.V., Goldberg E.M., Goli S., Gómez-Dantés H., Gona P.N., Gopalani S.V., Gorman T.M., Goulart A.C., Goulart B.N.G., Grada A., Grams M.E., Grosso G., Gugnani H.C., Guo Y., Gupta P.C., Gupta Rahul, Gupta Rajeev, Gupta T., Gyawali B., Haagsma J.A., Hachinski V., Hafezi-Nejad N., Haghparast Bidgoli H., Hagos T.B., Hailu G.B., Haj-Mirzaian Arvin, Haj-Mirzaian Arya, Hamadeh R.R., Hamidi S., Handal A.J., Hankey G.J., Hao Y., Harb H.L., Harikrishnan S., Haro J.M., Hasan M., Hassankhani H., Hassen H.Y., Havmoeller R., Hawley C.N., Hay R.J., Hay S.I., Hedayatizadeh-Omran A., Heibati B., Hendrie D., Henok A., Herteliu C., Heydarpour S., Hibstu D.T., Hoang H.T., Hoek H.W., Hoffman H.J., Hole M.K., Homaie Rad E., Hoogar P., Hosgood H.D., Hosseini S.M., Hosseinzadeh M., Hostiuc M., Hostiuc S., Hotez P.J., Hoy D.G., Hsairi M., Htet A.S., Hu G., Huang J.J., Huynh C.K., Iburg K.M., Ikeda C.T., Ileanu B., Ilesanmi O.S., Iqbal U., Irvani S.S.N., Irvine C.M.S., Islam S.M.S., Islami F., Jacobsen K.H., Jahangiry L., Jahanmehr N., Jain S.K., Jakovljevic M., Javanbakht M., Jayatilleke A.U., Jeemon P., Jha R.P., Jha V., Ji J.S., Johnson C.O., Jonas J.B., Jozwiak J.J., Jungari S.B., Jürisson M., Kabir Z., Kadel R., Kahsay A., Kalani R., Kanchan T., Karami M., Karami Matin B., Karch A., Karema C., Karimi N., Karimi S.M., Kasaeian A., Kassa D.H., Kassa G.M., Kassa T.D., Kassebaum N.J., Katikireddi S.V., Kawakami N., Karyani A.K., Keighobadi M.M., Keiyoro P.N., Kemmer L., Kemp G.R., Kengne A.P., Keren A., Khader Y.S., Khafaei B., Khafaie M.A., Khajavi A., Khalil I.A., Khan E.A., Khan M.S., Khan M.A., Khang Y.-H., Khazaei M., Khoja A.T., Khosravi A., Khosravi M.H., Kiadaliri A.A., Kiirithio D.N., Kim C.-I., Kim D., Kim P., Kim Y.-E., Kim Y.J., Kimokoti R.W., Kinfu Y., Kisa A., Kissimova-Skarbek K., Kivimäki M., Knudsen A.K.S., Kocarnik J.M., Kochhar S., Kokubo Y., Kolola T., Kopec J.A., Kosen S., Kotsakis G.A., Koul P.A., Koyanagi A., Kravchenko M.A., Krishan K., Krohn K.J., Kuate Defo B., Kucuk Bicer B., Kumar G.A., Kumar M., Kyu H.H., Lad D.P., Lad S.D., Lafranconi A., Lalloo R., Lallukka T., Lami F.H., Lansingh V.C., Latifi A., Lau K.M.-M., Lazarus J.V., Leasher J.L., Ledesma J.R., Lee P.H., Leigh J., Leung J., Levi M., Lewycka S., Li S., Li Y., Liao Y., Liben M.L., Lim L.-L., Lim S.S., Liu S., Lodha R., Looker K.J., Lopez A.D., Lorkowski S., Lotufo P.A., Low N., Lozano R., Lucas T.C.D., Lucchesi L.R., Lunevicius R., Lyons R.A., Ma S., Macarayan E.R.K., Mackay M.T., Madotto F., Magdy Abd El Razek H., Magdy Abd El Razek M., Maghavani D.P., Mahotra N.B., Mai H.T., Majdan M., Majdzadeh R., Majeed A., Malekzadeh R., Malta D.C., Mamun A.A., Manda A.-L., Manguerra H., Manhertz T., Mansournia M.A., Mantovani L.G., Mapoma C.C., Maravilla J.C., Marcenes W., Marks A., Martins-Melo F.R., Martopullo I., März W., Marzan M.B., Mashamba-Thompson T.P., Massenburg B.B., Mathur M.R., Matsushita K., Maulik P.K., Mazidi M., McAlinden C., McGrath J.J., McKee M., Mehndiratta M.M., Mehrotra R., Mehta K.M., Mehta V., Mejia-Rodriguez F., Mekonen T., Melese A., Melku M., Meltzer M., Memiah P.T.N., Memish Z.A., Mendoza W., Mengistu D.T., Mengistu G., Mensah G.A., Mereta S.T., Meretoja A., Meretoja T.J., Mestrovic T., Mezerji N.M.G., Miazgowski B., Miazgowski T., Millear A.I., Miller T.R., Miltz B., Mini G.K., Mirarefin M., Mirrakhimov E.M., Misganaw A.T., Mitchell P.B., Mitiku H., Moazen B., Mohajer B., Mohammad K.A., Mohammadifard N., Mohammadnia-Afrouzi M., Mohammed M.A., Mohammed S., Mohebi F., Moitra M., Mokdad A.H., Molokhia M., Monasta L., Moodley Y., Moosazadeh M., Moradi G., Moradi-Lakeh M., Moradinazar M., Moraga P., Morawska L., Moreno Velásquez I., Morgado-Da-Costa J., Morrison S.D., Moschos M.M., Mountjoy-Venning W.C., Mousavi S.M., Mruts K.B., Muche A.A., Muchie K.F., Mueller U.O., Muhammed O.S., Mukhopadhyay S., Muller K., Mumford J.E., Murhekar M., Musa J., Musa K.I., Mustafa G., Nabhan A.F., Nagata C., Naghavi M., Naheed A., Nahvijou A., Naik G., Naik N., Najafi F., Naldi L., Nam H.S., Nangia V., Nansseu J.R., Nascimento B.R., Natarajan G., Neamati N., Negoi I., Negoi R.I., Neupane S., Newton C.R.J., Ngunjiri J.W., Nguyen A.Q., Nguyen HaThu, Nguyen H.L.T., Nguyen Huong Thanh, Nguyen L.H., Nguyen M., Nguyen N.B., Nguyen S.H., Nichols E., Ningrum D.N.A., Nixon M.R., Nolutshungu N., Nomura S., Norheim O.F., Noroozi M., Norrving B., Noubiap J.J., Nouri H.R., Nourollahpour Shiadeh M., Nowroozi M.R., Nsoesie E.O., Nyasulu P.S., Odell C.M., Ofori-Asenso R., Ogbo F.A., Oh I.-H., Oladimeji O., Olagunju A.T., Olagunju T.O., Olivares P.R., Olsen H.E., Olusanya B.O., Ong K.L., Ong S.K., Oren E., Ortiz A., Ota E., Otstavnov S.S., Øverland S., Owolabi M.O., Pa M., Pacella R., Pakpour A.H., Pana A., Panda-Jonas S., Parisi A., Park E.-K., Parry C.D.H., Patel S., Pati S., Patil S.T., Patle A., Patton G.C., Paturi V.R., Paulson K.R., Pearce N., Pereira D.M., Perico N., Pesudovs K., Pham H.Q., Phillips M.R., Pigott D.M., Pillay J.D., Piradov M.A., Pirsaheb M., Pishgar F., Plana-Ripoll O., Plass D., Polinder S., Popova S., Postma M.J., Pourshams A., Poustchi H., Prabhakaran D., Prakash S., Prakash V., Purcell C.A., Purwar M.B., Qorbani M., Quistberg D.A., Radfar A., Rafay A., Rafiei A., Rahim F., Rahimi K., Rahimi-Movaghar A., Rahimi-Movaghar V., Rahman M., Rahman M.H.ur, Rahman M.A., Rahman S.U., Rai R.K., Rajati F., Ram U., Ranjan P., Ranta A., Rao P.C., Rawaf D.L., Rawaf S., Reddy K.S., Reiner R.C., Reinig N., Reitsma M.B., Remuzzi G., Renzaho A.M.N., Resnikoff S., Rezaei S., Rezai M.S., Ribeiro A.L.P., Roberts N.L.S., Robinson S.R., Roever L., Ronfani L., Roshandel G., Rostami A., Roth G.A., Roy A., Rubagotti E., Sachdev P.S., Sadat N., Saddik B., Sadeghi E., Saeedi Moghaddam S., Safari H., Safari Y., Safari-Faramani R., Safdarian M., Safi S., Safiri S., Sagar R., Sahebkar A., Sahraian M.A., Sajadi H.S., Salam N., Salama J.S., Salamati P., Saleem K., Saleem Z., Salimi Y., Salomon J.A., Salvi S.S., Salz I., Samy A.M., Sanabria J., Sang Y., Santomauro D.F., Santos I.S., Santos J.V., Santric Milicevic M.M., Sao Jose B.P., Sardana M., Sarker A.R., Sarrafzadegan N., Sartorius B., Sarvi S., Sathian B., Satpathy M., Sawant A.R., Sawhney M., Saxena S., Saylan M., Schaeffner E., Schmidt M.I., Schneider I.J.C., Schöttker B., Schwebel D.C., Schwendicke F., Scott J.G., Sekerija M., Sepanlou S.G., Serván-Mori E., Seyedmousavi S., Shabaninejad H., Shafieesabet A., Shahbazi M., Shaheen A.A., Shaikh M.A., Shams-Beyranvand M., Shamsi M., Shamsizadeh M., Sharafi H., Sharafi K., Sharif M., Sharif-Alhoseini M., Sharma M., Sharma R., She J., Sheikh A., Shi P., Shibuya K., Shigematsu M., Shiri R., Shirkoohi R., Shishani K., Shiue I., Shokraneh F., Shoman H., Shrime M.G., Si S., Siabani S., Siddiqi T.J., Sigfusdottir I.D., Sigurvinsdottir R., Silva J.P., Silveira D.G.A., Singam N.S.V., Singh J.A., Singh N.P., Singh V., Sinha D.N., Skiadaresi E., Slepak E.L.N., Sliwa K., Smith D.L., Smith M., Soares Filho A.M., Sobaih B.H., Sobhani S., Sobngwi E., Soneji S.S., Soofi M., Soosaraei M., Sorensen R.J.D., Soriano J.B., Soyiri I.N., Sposato L.A., Sreeramareddy C.T., Srinivasan V., Stanaway J.D., Stein D.J., Steiner C., Steiner T.J., Stokes M.A., Stovner L.J., Subart M.L., Sudaryanto A., Sufiyan M.B., Sunguya B.F., Sur P.J., Sutradhar I., Sykes B.L., Sylte D.O., Tabarés-Seisdedos R., Tadakamadla S.K., Tadesse B.T., Tandon N., Tassew S.G., Tavakkoli M., Taveira N., Taylor H.R., Tehrani-Banihashemi A., Tekalign T.G., Tekelemedhin S.W., Tekle M.G., Temesgen H., Temsah M.-H., Temsah O., Terkawi A.S., Teweldemedhin M., Thankappan K.R., Thomas N., Tilahun B., To Q.G., Tonelli M., Topor-Madry R., Topouzis F., Torre A.E., Tortajada-Girbés M., Touvier M., Tovani-Palone M.R., Towbin J.A., Tran B.X., Tran K.B., Troeger C.E., Truelsen T.C., Tsilimbaris M.K., Tsoi D., Tudor Car L., Tuzcu E.M., Ukwaja K.N., Ullah I., Undurraga E.A., Unutzer J., Updike R.L., Usman M.S., Uthman O.A., Vaduganathan M., Vaezi A., Valdez P.R., Varughese S., Vasankari T.J., Venketasubramanian N., Villafaina S., Violante F.S., Vladimirov S.K., Vlassov V., Vollset S.E., Vosoughi K., Vujcic I.S., Wagnew F.S., Waheed Y., Waller S.G., Wang Y., Wang Y.-P., Weiderpass E., Weintraub R.G., Weiss D.J., Weldegebreal F., Weldegwergs K.G., Werdecker A., West T.E., Whiteford H.A., Widecka J., Wijeratne T., Wilner L.B., Wilson S., Winkler A.S., Wiyeh A.B., Wiysonge C.S., Wolfe C.D.A., Woolf A.D., Wu S., Wu Y.-C., Wyper G.M.A., Xavier D., Xu G., Yadgir S., Yadollahpour A., Yahyazadeh Jabbari S.H., Yamada T., Yan L.L., Yano Y., Yaseri M., Yasin Y.J., Yeshaneh A., Yimer E.M., Yip P., Yisma E., Yonemoto N., Yoon S.-J., Yotebieng M., Younis M.Z., Yousefifard M., Yu C., Zadnik V., Zaidi Z., Zaman S.B., Zamani M., Zare Z., Zeleke A.J., Zenebe Z.M., Zhang K., Zhao Z., Zhou M., Zodpey S., Zucker I., Vos T., Murray C.J.L. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J., Levens S.M., Gotlib I.H. Sticky thoughts: depression and rumination are associated with difficulties manipulating emotional material in working memory. Psychol Sci. 2011;22:979–983. doi: 10.1177/0956797611415539. 0956797611415539 [pii] [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Spielberg J.M., Warren S.L., Sutton B.P., Miller G.A., Heller W., Banich M.T. Distracted and down: neural mechanisms of affective interference in subclinical depression. Soc. Cogn. Affect. Neurosci. 2015;10:654–663. doi: 10.1093/scan/nsu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Whitfield-Gabrieli S., Dillon D.G., Goer F., Beltzer M., Minkel J., Smoski M., Dichter G., Pizzagalli D.A. Dynamic Resting-State Functional Connectivity in Major Depression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016;41:1822–1830. doi: 10.1038/npp.2015.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Bowman F.D., Mayberg H., Liu H. A depression network of functionally connected regions discovered via multi-attribute canonical correlation graphs. NeuroImage. 2016;141:431–441. doi: 10.1016/j.neuroimage.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahanoglu F.I., Bayram I., Van De Ville D. A Signal Processing Approach to Generalized 1-D Total Variation. IEEE Transactions on Signal Processing. 2011;59(11):5265–5274. [Google Scholar]
- Karahanoğlu F.I., Caballero-Gaudes C., Lazeyras F., Van de Ville D. Total activation: fMRI deconvolution through spatio-temporal regularization. NeuroImage. 2013;73:121–134. doi: 10.1016/j.neuroimage.2013.01.067. [DOI] [PubMed] [Google Scholar]
- Karahanoğlu F.I., Van De Ville D. Dynamics of large-scale fMRI networks: Deconstruct brain activity to build better models of brain function. Curr. Opin. Biomed. Eng. New Developments in Biomedical Imaging. 2017;3:28–36. doi: 10.1016/j.cobme.2017.09.008. [DOI] [Google Scholar]
- Karahanoğlu, F.I., Ville, D.V.D., 2016. Total-activation regularized deconvolution of resting-state fMRI leads to reproducible networks with spatial overlap, in: 2016 24th European Signal Processing Conference (EUSIPCO). Presented at the 2016 24th European Signal Processing Conference (EUSIPCO), pp. 260–264. 10.1109/EUSIPCO.2016.7760250.
- Karahanoğlu F.I., Ville D.V.D. Transient brain activity disentangles fMRI resting-state dynamics in terms of spatially and temporally overlapping networks. Nat. Commun. 2015;6:7751. doi: 10.1038/ncomms8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C., Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends Cogn. Sci. 2007;11:194–196. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Khadka S., Meda S.A., Stevens M.C., Glahn D.C., Calhoun V.D., Sweeney J.A., Tamminga C.A., Keshavan M.S., O’Neil K., Schretlen D., Pearlson G.D. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol. Psychiatry. 2013;74:458–466. doi: 10.1016/j.biopsych.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Katovich K., Suri G. Inferring affect from fMRI data. Trends Cogn. Sci. 2014;18:422–428. doi: 10.1016/j.tics.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Koenigsberg H.W., Denny B.T., Fan J., Liu X., Guerreri S., Mayson S.J., Rimsky L., New A.S., Goodman M., Siever L.J. The neural correlates of anomalous habituation to negative emotional pictures in borderline and avoidant personality disorder patients. Am. J. Psychiatry. 2014;171:82–90. doi: 10.1176/appi.ajp.2013.13070852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H., Minamoto T., Yaoi K., Osaka M., Osaka N. Coactivation of the Default Mode Network regions and Working Memory Network regions during task preparation. Sci. Rep. 2014;4:5954. doi: 10.1038/srep05954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S., Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr. Bull. 2013;39:358–365. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker S.A., Jacobs R.H., Passarotti A.M. Current Neural and Behavioral Dimensional Constructs across Mood Disorders. Curr. Behav. Neurosci. Rep. 2014;1:144–153. doi: 10.1007/s40473-014-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Kamourieh S., Beckmann C.F., Sharp D.J. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C., Gorwood P., Bergouignan L., Pélissolo A., Lehéricy S., Fossati P. Negative affectivity, self-referential processing and the cortical midline structures. Soc. Cogn. Affect. Neurosci. 2011;6:426–433. doi: 10.1093/scan/nsq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi N., Richiardi J., Gschwind M., Simioni S., Annoni J.-M., Schluep M., Vuilleumier P., Van De Ville D. Principal components of functional connectivity: a new approach to study dynamic brain connectivity during rest. NeuroImage. 2013;83:937–950. doi: 10.1016/j.neuroimage.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Li B., Liu L., Friston K.J., Shen H., Wang L., Zeng L.-L., Hu D. A treatment-resistant default mode subnetwork in major depression. Biol. Psychiatry. 2013;74:48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Liu C., Pu W., Wu G., Zhao J., Xue Z. Abnormal resting-state cerebral-limbic functional connectivity in bipolar depression and unipolar depression. BMC Neurosci. 2019;20:30. doi: 10.1186/s12868-019-0508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-H., Ma X., Wu X., Zhang Y., Zhou F.-C., Li F., Tie C.-L., Dong J., Wang Y.-J., Yang Z., Wang C.-Y. Regional homogeneity of resting-state brain abnormalities in bipolar and unipolar depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;41:52–59. doi: 10.1016/j.pnpbp.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Liu X., Duyn J.H. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4392–4397. doi: 10.1073/pnas.1216856110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wu X., Zhang J., Guo X., Long Z., Yao L. Altered effective connectivity model in the default mode network between bipolar and unipolar depression based on resting-state fMRI. J. Affect. Disord. 2015;182:8–17. doi: 10.1016/j.jad.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Lois G., Wessa M. Differential association of default mode network connectivity and rumination in healthy individuals and remitted MDD patients. Soc. Cogn. Affect. Neurosci. 2016;11:1792–1801. doi: 10.1093/scan/nsw085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y., Cao H., Yan C., Chen X., Li L., Castellanos F.X., Bai T., Bo Q., Chen G., Chen N., Chen W., Cheng C., Cheng Y., Cui X., Duan J., Fang Y., Gong Q., Guo W., Hou Z., Hu L., Kuang L., Li F., Li K., Li T., Liu Y., Luo Q., Meng H., Peng D., Qiu H., Qiu J., Shen Y., Shi Y., Si T., Wang C., Wang F., Wang K., Wang L., Wang X., Wang Y., Wu X., Wu X., Xie C., Xie G., Xie H., Xie P., Xu X., Yang H., Yang J., Yao J., Yao S., Yin Y., Yuan Y., Zhang A., Zhang H., Zhang K., Zhang L., Zhang Z., Zhou R., Zhou Y., Zhu J., Zou C., Zang Y., Zhao J., Kin-yuen Chan C., Pu W., Liu Z. Altered resting-state dynamic functional brain networks in major depressive disorder: Findings from the REST-meta-MDD consortium. NeuroImage Clin. 2020;26 doi: 10.1016/j.nicl.2020.102163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., Chen G., Jia Y., Zhong S., Gong J., Chen F., Wang J., Qi Z., Liu X., Huang L., Wang Y. Shared and specific dynamics of brain segregation and integration in bipolar disorder and major depressive disorder: A resting-state functional magnetic resonance imaging study. J. Affect. Disord. 2021;280:279–286. doi: 10.1016/j.jad.2020.11.012. [DOI] [PubMed] [Google Scholar]
- Ma X., Liu J., Liu T., Ma L., Wang W., Shi S., Wang Y., Gong Q., Wang M. Altered Resting-State Functional Activity in Medication-Naive Patients With First-Episode Major Depression Disorder vs. Healthy Control: A Quantitative Meta-Analysis. Front. Behav. Neurosci. 2019;13:89. doi: 10.3389/fnbeh.2019.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A., Meng C., Brandl F., Doll A., Tahmasian M., Scherr M., Schwerthöffer D., Zimmer C., Förstl H., Bäuml J., Riedl V., Wohlschläger A.M., Sorg C. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci. 2013;7:930. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D., Perrucci M.G., Del Gratta C., Romani G.L., Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti I., Koster E.H.W., Sonuga-Barke E.J., De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol. Rev. 2012;22:229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- Margulies D.S., Vincent J.L., Kelly C., Lohmann G., Uddin L.Q., Biswal B.B., Villringer A., Castellanos F.X., Milham M.P., Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc. Natl. Acad. Sci. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino M., Magioncalda P., Huang Z., Conio B., Piaggio N., Duncan N.W., Rocchi G., Escelsior A., Marozzi V., Wolff A., Inglese M., Amore M., Northoff G. Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc. Natl. Acad. Sci. U. S. A. 2016;113:4824–4829. doi: 10.1073/pnas.1517558113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda S.A., Ruano G., Windemuth A., O’Neil K., Berwise C., Dunn S.M., Boccaccio L.E., Narayanan B., Kocherla M., Sprooten E., Keshavan M.S., Tamminga C.A., Sweeney J.A., Clementz B.A., Calhoun V.D., Pearlson G.D. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E2066–E2075. doi: 10.1073/pnas.1313093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milazzo A.-C., Ng B., Jiang H., Shirer W., Varoquaux G., Poline J.B., Thirion B., Greicius M.D. Identification of Mood-Relevant Brain Connections Using a Continuous, Subject-Driven Rumination Paradigm. Cereb. Cortex N. Y. N. 2016;1991(26):933–942. doi: 10.1093/cercor/bhu255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry J. Ment. Sci. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Mulders P.C., van Eijndhoven P.F., Schene A.H., Beckmann C.F., Tendolkar I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci. Biobehav. Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Okada N., Koshiyama D., Kamiya K., Abe O., Kunimatsu A., Okanoya K., Kasai K., Koike S. Differences in Functional Connectivity Networks Related to the Midbrain Dopaminergic System-Related Area in Various Psychiatric Disorders. Schizophr. Bull. 2020 doi: 10.1093/schbul/sbz121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. J. Pers. Soc. Psychol. 1991;61:115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Palmer S.M., Crewther S.G., Carey L.M., START Project Team A meta-analysis of changes in brain activity in clinical depression. Front. Hum. Neurosci. 2014;8:1045. doi: 10.3389/fnhum.2014.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B. Interoception in anxiety and depression. Brain Struct. Funct. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellet, J., Bobon, D.P., Mormont, I., Lang, F., Massardier, A., 1980. Etude Princeps de la Validation Française de la MADRS, Sous-Echelle Dépression de la CPRS.
- Peters A.T., Burkhouse K., Feldhaus C.C., Langenecker S.A., Jacobs R.H. Aberrant resting-state functional connectivity in limbic and cognitive control networks relates to depressive rumination and mindfulness: A pilot study among adolescents with a history of depression. J. Affect. Disord. 2016;200:178–181. doi: 10.1016/j.jad.2016.03.059. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry. 2008;13(829):833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichot P., Pull C.B., von Frenckell R., Pull M.-C. A factorial analysis of the Hamilton Anxiety Rating Scale. Psychiatr. Fenn. 1981;183 [Google Scholar]
- Piguet C., Cojan Y., Sterpenich V., Desseilles M., Bertschy G., Vuilleumier P. Alterations in neural systems mediating cognitive flexibility and inhibition in mood disorders. Hum. Brain Mapp. 2016;37:1335–1348. doi: 10.1002/hbm.23104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet C., Desseilles M., Sterpenich V., Cojan Y., Bertschy G., Vuilleumier P. Neural substrates of rumination tendency in non-depressed individuals. Biol. Psychol. 2014;103:195–202. doi: 10.1016/j.biopsycho.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Pillai A.S., Jirsa V.K. Symmetry Breaking in Space-Time Hierarchies Shapes Brain Dynamics and Behavior. Neuron. 2017;94:1010–1026. doi: 10.1016/j.neuron.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Poldrack R. Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Preti M.G., Bolton T.A., Van De Ville D. The dynamic functional connectome: State-of-the-art and perspectives. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.12.061. [DOI] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Rey, G., Bolton, T.A.W., Gaviria, J., Piguet, C., Preti, M.G., Favre, S., Aubry, J.-M., Ville, D.V.D., Vuilleumier, P., 2021. Dynamics of amygdala connectivity in bipolar disorders: A longitudinal study across mood states. medRxiv 2021.03.30.21254608. doi: 10.1101/2021.03.30.21254608. [DOI] [PMC free article] [PubMed]
- Rey G., Piguet C., Benders A., Favre S., Eickhoff S.B., Aubry J.-M., Vuilleumier P. Resting-state functional connectivity of emotion regulation networks in euthymic and non-euthymic bipolar disorder patients. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2016;34:56–63. doi: 10.1016/j.eurpsy.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Sambataro F., Wolf N.D., Pennuto M., Vasic N., Wolf R.C. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychol. Med. 2014;44:2041–2051. doi: 10.1017/S0033291713002596. [DOI] [PubMed] [Google Scholar]
- Schaefer A., Margulies D.S., Lohmann G., Gorgolewski K.J., Smallwood J., Kiebel S.J., Villringer A. Dynamic network participation of functional connectivity hubs assessed by resting-state fMRI. Front. Hum. Neurosci. 2014;8:195. doi: 10.3389/fnhum.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R.E., Gay P., Courvoisier D., Jermann F., Ceschi G., David M., Brinkmann K., Van der Linden M. Anatomy of the White Bear Suppression Inventory (WBSI): a review of previous findings and a new approach. J. Pers. Assess. 2009;91:323–330. doi: 10.1080/00223890902935738. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20) pp. 22–33;quiz 34–57. PMID: 9881538. [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N., Snyder A.Z., Mintun M.A., Wang S., Coalson R.S., Raichle M.E. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z., Mintun M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. 1000446107 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig D., Loscalzo J. Precision Psychiatry Meets Network Medicine: Network Psychiatry. JAMA Psychiatry. 2017;74:665–666. doi: 10.1001/jamapsychiatry.2017.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.K., Kelley R.G., Chang K.D., Gotlib I.H. Intrinsic Amygdala Functional Connectivity in Youth With Bipolar I Disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:763–770. doi: 10.1016/j.jaac.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Miller K.L., Moeller S., Xu J., Auerbach E.J., Woolrich M.W., Beckmann C.F., Jenkinson M., Andersson J., Glasser M.F., Van Essen D.C., Feinberg D.A., Yacoub E.S., Ugurbil K. Temporally-independent functional modes of spontaneous brain activity. Proc. Natl. Acad. Sci. U. S. A. 2012;109:3131–3136. doi: 10.1073/pnas.1121329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg J.M., Beall E.B., Hulvershorn L.A., Altinay M., Karne H., Anand A. Resting State Brain Network Disturbances Related to Hypomania and Depression in Medication-Free Bipolar Disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016;41:3016–3024. doi: 10.1038/npp.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J., Tseng W.-L., Kim P., Chen G., Yi J., Donahue L., Brotman M.A., Towbin K.E., Pine D.S., Leibenluft E. Association of Irritability and Anxiety With the Neural Mechanisms of Implicit Face Emotion Processing in Youths With Psychopathology. JAMA Psychiatry. 2016 doi: 10.1001/jamapsychiatry.2016.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski S.M., Adler C.M., Almeida J., Altshuler L.L., Blumberg H.P., Chang K.D., DelBello M.P., Frangou S., McIntosh A., Phillips M.L., Sussman J.E., Townsend J.D. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann B., Olde lütke Beverborg M., Pfleiderer B. Toward literature-based feature selection for diagnostic classification: a meta-analysis of resting-state fMRI in depression. Front. Hum. Neurosci. 2014;8:692. doi: 10.3389/fnhum.2014.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syan S.K., Smith M., Frey B.N., Remtulla R., Kapczinski F., Hall G.B.C., Minuzzi L. Resting-state functional connectivity in individuals with bipolar disorder during clinical remission: a systematic review. J. Psychiatry Neurosci. JPN. 2018;43 doi: 10.1503/jpn.170175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Kong L., Wu F., Womer F., Jiang W., Cao Y., Ren L., Wang J., Fan G., Blumberg H.P., Xu K., Wang F. Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: a resting-state functional magnetic resonance imaging study. Psychol. Med. 2013;43:1921–1927. doi: 10.1017/S0033291712002759. [DOI] [PubMed] [Google Scholar]
- Teng S., Lu C.-F., Wang P.-S., Li C.-T., Tu P.-C., Hung C.-I., Su T.-P., Wu Y.-T. Altered resting-state functional connectivity of striatal-thalamic circuit in bipolar disorder. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0096422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W., Gonzalez R., Nolen-Hoeksema S. Rumination Reconsidered: A Psychometric Analysis. Cogn. Ther. Res. 2003;27:247–259. [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vargas C., López-Jaramillo C., Vieta E. A systematic literature review of resting state network–functional MRI in bipolar disorder. J. Affect. Disord. 2013;150:727–735. doi: 10.1016/j.jad.2013.05.083. [DOI] [PubMed] [Google Scholar]
- Vidaurre D., Smith S.M., Woolrich M.W. Brain network dynamics are hierarchically organized in time. Proc. Natl. Acad. Sci. 2017;114:12827–12832. doi: 10.1073/pnas.1705120114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Gao Y., Tang S., Lu L., Zhang L., Bu X., Li H., Hu X., Hu X., Jiang P., Jia Z., Gong Q., Sweeney J.A., Huang X. Large-scale network dysfunction in the acute state compared to the remitted state of bipolar disorder: A meta-analysis of resting-state functional connectivity. EBioMedicine. 2020;54 doi: 10.1016/j.ebiom.2020.102742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M., Qin J., Yan R., Bi K., Liu C., Yao Z., Lu Q. Abnormal dynamic community structure of the salience network in depression. J. Magn. Reson. Imaging JMRI. 2016 doi: 10.1002/jmri.25429. [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.-G., Chen X., Li L., Castellanos F.X., Bai T.-J., Bo Q.-J., Cao J., Chen G.-M., Chen N.-X., Chen W., Cheng C., Cheng Y.-Q., Cui X.-L., Duan J., Fang Y.-R., Gong Q.-Y., Guo W.-B., Hou Z.-H., Hu L., Kuang L., Li F., Li K.-M., Li T., Liu Y.-S., Liu Z.-N., Long Y.-C., Luo Q.-H., Meng H.-Q., Peng D.-H., Qiu H.-T., Qiu J., Shen Y.-D., Shi Y.-S., Wang C.-Y., Wang F., Wang K., Wang L., Wang X., Wang Y., Wu X.-P., Wu X.-R., Xie C.-M., Xie G.-R., Xie H.-Y., Xie P., Xu X.-F., Yang H., Yang J., Yao J.-S., Yao S.-Q., Yin Y.-Y., Yuan Y.-G., Zhang A.-X., Zhang H., Zhang K.-R., Zhang L., Zhang Z.-J., Zhou R.-B., Zhou Y.-T., Zhu J.-J., Zou C.-J., Si T.-M., Zuo X.-N., Zhao J.-P., Zang Y.-F. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc. Natl. Acad. Sci. 2019;116:9078–9083. doi: 10.1073/pnas.1900390116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Qing P., Zhang K., Liu G. Altered network efficiency in major depressive disorder. BMC Psychiatry. 2016;16:450. doi: 10.1186/s12888-016-1053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T.T., Krienen F.M., Chee M.W.L., Buckner R.L. Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. NeuroImage. 2013;88C:212–227. doi: 10.1016/j.neuroimage.2013.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zöllei L., Polimeni J.R., Fischl B., Liu H., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Qin J., Xiong X., Xu F., Wang J., Hou F., Yang A. Abnormal topology of brain functional networks in unipolar depression and bipolar disorder using optimal graph thresholding. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2020;96 doi: 10.1016/j.pnpbp.2019.109758. [DOI] [PubMed] [Google Scholar]
- Zeng C., Xue Z., Ross B., Zhang M., Liu Z., Wu G., Ouyang X., Li D., Pu W. Salience-thalamic circuit uncouples in major depressive disorder, but not in bipolar depression. J. Affect. Disord. 2020;269:43–50. doi: 10.1016/j.jad.2020.03.007. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang R., Han S., Womer F.Y., Wei Y., Duan J., Chang M., Li C., Feng R., Liu J., Zhao P., Jiang X., Wei S., Yin Z., Zhang Y., Zhang Y., Zhang X., Tang Y., Wang F. Three major psychiatric disorders share specific dynamic alterations of intrinsic brain activity. Schizophr. Res. 2021 doi: 10.1016/j.schres.2021.06.014. [DOI] [PubMed] [Google Scholar]
- Zhou H.-X., Chen X., Shen Y.-Q., Li L., Chen N.-X., Zhu Z.-C., Castellanos F.X., Yan C.-G. Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. NeuroImage. 2020;206 doi: 10.1016/j.neuroimage.2019.116287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.