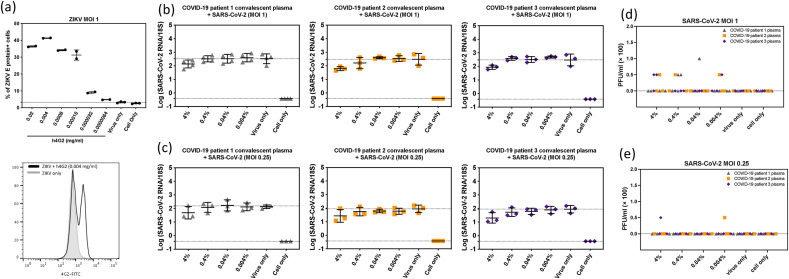
Fig. 2.
Antibody-dependent enhancement (ADE) effect of COVID-19 convalescent plasma in primary human monocyte derived macrophages. (a) Representative figure and histogram plot of intracellular ZIKV E protein showing ADE induced by humanized 4G2 antibody. This assay served as a positive control in these experiments. Intracellular viral E protein was increased in human macrophages when ZIKV was incubated with various concentrations of humanized 4G2 antibody prior to inoculation onto macrophages. ADE of SARS-CoV-2 was evaluated by changes in intracellular viral genomic RNA in macrophages and extracellular infectious particles released in culture supernatant. Viral RNA (b, c) and infectious viral particles (d, e) at 2 days post infection with SARS-CoV-2 pre-incubated with serial diluted COVID-19 patient convalescent plasma at MOI 1 (b, d) and MOI 0.25 (c, e). These data are shown as the mean with SD (n = 3–4). Each data point represents the average of a duplicate of each testing condition with individual donor macrophages. One-Way ANOVA was used to test the difference of viral titers between various concentration of patient plasma and virus only group.