Abstract
Liver-type fatty acid-binding protein (L-FABP) is a biomarker for the early detection of renal diseases in humans. L-FABP is a cytotoxic oxidation product secreted from the proximal tubules under ischemic and oxidative stress conditions. First, L-FABP gene expression in the kidney and liver was evaluated. Next, the urinary L-FABP concentrations in dogs with or without renal diseases were measured using a novel enzyme-linked immunosorbent assay kit. Urinary L-FABP was normalized relative to urinary creatinine (uCre) concentrations (µg/g uCre). Finally, the relationships between urinary L-FABP and renal biomarkers used in canine medicine or serum alanine transaminase (ALT) as an indicator of liver damage were examined. Serum and urine samples from 94 client-owned dogs including 23 dogs with renal diseases and 71 dogs without renal diseases were used for analysis. Relative L-FABP gene expression was confirmed both in the liver and kidney. Dogs with renal diseases had a significantly higher urinary L-FABP than those without, and its predictive cutoff value was 26 µg/g uCre. Urinary L-FABP was significantly correlated with serum creatinine (r=0.4674, P<0.01), urea nitrogen (r=0.4907, P<0.01), urine specific gravity (r=−0.5100, P<0.01), and urine protein/creatinine ratio (r=0.7216, P<0.01), but not with serum ALT. Hence, dogs with a high urinary L-FABP value were more likely to have renal diseases.
Keywords: dog, renal disease, urinary liver-type fatty acid-binding protein
In canine medicine, tubular damage is indirectly evaluated using urinary specific gravity and osmolality. The human biomarkers for renal tubular function or damage include beta-2-microglobulin, alpha-1-microglobulin, and N-acetyl-beta-D-glucosaminidase, and the development of tubular biomarkers in canine medicine has gained considerable interest [22]. However, in veterinary medicine currently, biomarkers have been commonly used only in experiments or for research purposes and established and useful clinical markers have not been identified.
Liver-type fatty acid-binding protein (L-FABP) is a 14-kDa protein found in the liver, kidney, and small intestine in humans and rats [2, 5, 6, 24]. L-FABP binds to hydrophobic ligands, including fatty acids, and functions as an intracellular transporter [5, 26]. Previous studies on animal models and in vitro experiments showed that L-FABP expression increased under hypoxic and oxidative stress conditions [9, 18, 29]. This finding indicates that L-FABP plays an important cytoprotective role by potentially binding to oxidative fatty acids. In human kidneys, L-FABP is found in the cytoplasm of the proximal tubular cells [5, 17], and is rapidly released into the tubular lumen in response to ischemia or oxidative stress [12, 30]. In human L-FABP chromosomal transgenic mouse models, tubulointerstitial damage increased the expression of L-FABP in the proximal tubules and urinary excretion of L-FABP [8, 15]. Human patients with progressing kidney disease have high urinary L-FABP [13]. A previous study in dogs [23] examined the urinary excretion of L-FABP with gentamicin-induced acute kidney injury (AKI). Furthermore, in dogs with chronic kidney disease (CKD), tubulointerstitial damage, which progresses with continuous proteinuria, leads to a shorter survival [10], and urinary L-FABP may reflect the degree of tubulointerstitial damage. Therefore, the evaluation of urinary L-FABP may contribute to the diagnosis of renal diseases.
The current study aimed to evaluate the efficacy of urinary L-FABP in the diagnosis of renal diseases in dogs. In dogs, L-FABP gene expression has been confirmed in the liver [11], but not in the kidney. First, to identify L-FABP expression in the kidney, we evaluated L-FABP transcription levels both in the liver and kidney of clinically healthy dogs. Then, we measured the urinary L-FABP concentrations of dogs with renal diseases, such as CKD and AKI, and those without. Finally, the correlations between urinary L-FABP and renal biomarkers used in canine medicine at present, such as serum creatinine, serum urea nitrogen, urine specific gravity (USG), and urine protein/creatinine (UPC) ratio, or serum alanine transaminase (ALT), indicating liver damage, were assessed.
MATERIALS AND METHODS
Animals and total RNA extraction from tissues
Liver and kidney tissues used were derived from three mature healthy Beagle dogs euthanized for another study approved by the Animal Care and Use Committee for Animal Experimentation of Gifu University (approval number: H30-172). All dogs were assessed as healthy based on the results of a physical examination, complete blood count, and biochemical profile performed prior to the experiment. Liver and kidney tissues were immediately collected from the dogs after euthanizing them. Collected tissues were immediately submerged in RNAlater (Qiagen, Hilden, Germany) and stored at −30°C for use later. Total RNA samples were extracted from the collected tissues using a commercially available kit (RNeasy Plus Mini Kit, Qiagen) according to the manufacturer’s instructions. A cDNA sample was synthesized from the total RNA sample using reverse transcriptase (ReverTra Dash, Toyobo, Osaka, Japan).
Quantitative transcription analysis of L-FABP (Real-time RT-PCR)
For the quantitative transcription analysis of L-FABP, the oligonucleotide primer pair of canine L-FABP reported previously in the liver [11] was partially modified (Table 1), whereas the oligonucleotide primer pair of canine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene (Table 1). We performed quantitative PCR using the SYBR Premix Ex Taq II (Tli RNaseH Plus; TaKaRa Bio Inc., Kusatsu, Japan) with StepOnePlusTM Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) by relative quantification using the calibration curve method. PCR protocol comprised an initial denaturation step at 95°C for 30 sec, followed by 40 cycles of amplification involving denaturation at 95°C for 15 sec, and annealing and extension at 60°C for 30 sec. The specific amplification of each gene was confirmed by agarose gel electrophoresis and melting curve analysis.
Table 1. Oligonucleotide sequences used as primers for real-time RT-PCR.
| Name | Type | Sequences (5′-3′) |
|---|---|---|
| L-FABP | Forward | GCAGAGCCAGGAAAACTTTG |
| Reverse | GGTGATTATGTCGCCGTTG | |
| GAPDH | Forward | CACCAACTGCTTGGCTCCTC |
| Reverse | CGTCACGCCACATCTTCCCA |
L-FABP, liver-type fatty acid-binding protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Blood and urine samples
For the clinical examination of client-owned dogs at Gifu University Animal Medical Center, serum samples were obtained, and urine samples were collected via cystocentesis or catheterization. Then, these samples were stored at −80°C until analysis. The study was approved by the Clinical Animal Ethical Review Committee of Gifu University (approval number: E17001). In total, 94 dogs (n=44 females and n=50 males, with a mean age of 7.8 ± 4.6 years) were enrolled in this study. The breeds were Toy Poodle (n=15), Beagle (n=15), Miniature Dachshund (n=14), mixed breed (n=9), Papillon (n=6), Labrador Retriever (n=4), Shiba (n=4), Shih Tzu (n=4), Chihuahua (n=3), Welsh Corgi Pembroke (n=2), Pomeranian (n=2), Miniature Schnauzer (n=2), Miniature Pinscher (n=2), Yorkshire Terrier (n=2), Shetland Sheep Dog (n=2), Bernese Mountain Dog (n=2), Maltese (n=2), West Highland Terrier (n=1), French bulldog (n=1), English Bulldog (n=1), and Brussels Griffon (n=1). The dogs were clinically diagnosed based on medical interview, physical examination, urinalysis, CBC, plasma biochemistry, ultrasonography, and radiography. The 94 dogs were divided into four groups (renal disease, clinically healthy, extrarenal urological disease, and non-urological disease groups). The dogs with renal disease were defined as a patient presenting with persistent azotemia (serum creatinine: >1.4 mg/dl) or renal proteinuria (UPC: >0.5) in conjunction with renal imaging test and other post-clinical examinations. The dogs with extrarenal urological disease were defined as a patient presenting with abnormality in the urinary tract, other than the kidney. There were 24 dogs with renal diseases including CKD (n=20) and AKI (n=4). CKD was classified based on the International Renal Interest Society (IRIS) staging system: stage I (n=5), II (n=6), and III (n=9). Among dogs without renal disease, 23 were clinically healthy, 20 presented with extrarenal urological disease, and 27 had non-urological diseases. Some dogs presented with LUTD including bacterial cystitis (n=7), chronic cystitis (n=6), transitional cell carcinoma (n=4), hormone-related urinary incontinence (n=2), and ectopic ureter (n=1). Meanwhile, other dogs had non-urological disease such as portosystemic shunt (n=12), chronic hepatitis (n=3), gall bladder mucoceles (n=3), inflammatory bowel disease (n=2), common bile duct obstruction (n=1), cholelithiasis (n=1), hepatic lymphoma (n=1), hepatocellular carcinoma (n=1), mandibular squamous cell carcinoma (n=1), ovarian granulosa cell tumor (n=1), and pyometra (n=1).
Assays
The urinary L-FABP concentration was evaluated using a two-step sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Canine L-FABP ELISA HS kit, CMIC Holdings, Tokyo, Japan). The urinary L-FABP concentration of the dogs ranged from 2.734 to 175 ng/ml, and the detectable range of L-FABP was >1.4 ng/ml. The efficacy of the ELISA kit for canine urine samples was validated in our laboratory. After performing serial dilution of six urine samples, the L-FABP concentration was found to have an adequate linearity within the range of the tested dilution rates. In eight urine samples, recovery after the addition of recombinant human L-FABP ranged from 93.1% to 114.8%. The inter- and intra-assay coefficients of variation for the L-FABP assay were 4.1% and 4.4%, respectively. The stability of six samples, as assessed by performing six repeated freeze-thaw cycles, ranged from 2.7% to 8.3%, and the value was expressed as an inter-assay coefficient of variation. When the sample contained L-FABP concentration above the upper limit of detection for this kit (>175 ng/ml), it was measured after diluting the urine samples with the standard dilution solution (0 ng/ml). When the sample contained L-FABP concentration below the detectable limit (1.4 ng/ml), its measured values at 1.4 ng/ml were considered. Serum creatinine, urea nitrogen, ALT, urinary creatinine, and total protein concentrations were assessed using an autoanalyzer (JCA-BM6050, JEOL Ltd., Tokyo, Japan). USG was evaluated with the centrifuged supernatant of urine at room temperature with a urinometer for dogs and cats (PAL, ATAGO, Tokyo, Japan).
Statistical analysis
Urinary L-FABP and total protein concentrations were normalized relative to urinary creatinine (uCre) concentrations to prevent variations caused by urinary volume. Then, they were expressed as urinary L-FABP (µg/g uCre) and UPC ratio. All statistical analyzes were performed using Excel 2010 (Microsoft, Redmond, WA, USA) with the Statcel three add-in software (OMS Publishing, Saitama, Japan). The transcription levels of L-FABP mRNA are represented as means ± standard deviation (S.D.) and compared between the liver and kidney using Student’s t-test. In clinically healthy dogs, gender differences in the urinary L-FABP and correlation with age were evaluated using the Mann–Whitney U test and Spearman’s correlation coefficient, respectively. The urinary L-FABP among the disease groups were evaluated using the multiple comparison test (Steel–Dwass test) for non-parametric data. The receiver operating characteristic (ROC) curve was plotted for the urinary L-FABP value, and the area under the curve was calculated. The cutoff value for predicting renal disease was determined based on the shortest distance from the ROC curve to the upper-left corner of the graph and the sensitivity and specificity of the cutoff value were calculated. The relationships between urinary L-FABP and serum creatinine, serum urea nitrogen, serum ALT, USG, and UPC ratio were analyzed using the Spearman’s correlation coefficient. The correlation between UPC ratio and urinary L-FABP only in dogs without urinary sediment (n=77) was analyzed. P values <0.05 were considered statistically significant.
RESULTS
Relative L-FABP RNA expression level in the liver and kidney
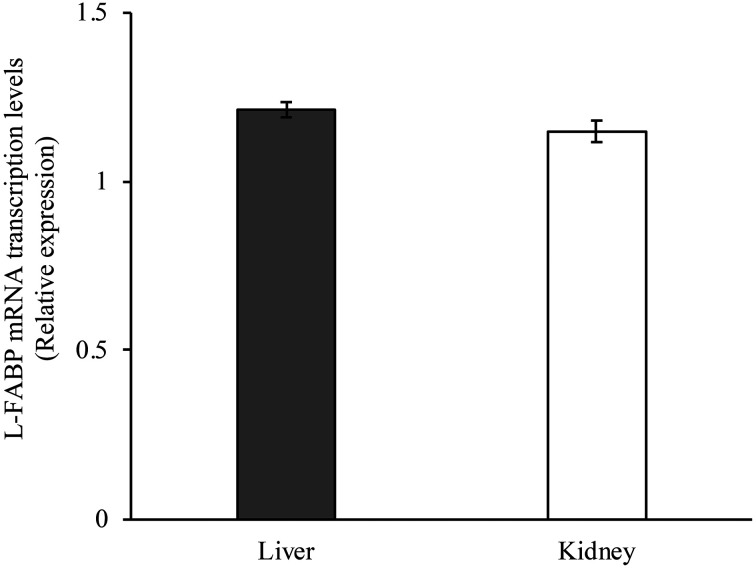
Relative L-FABP gene expression was confirmed both in the liver and kidney. There was no difference in the L-FABP expression levels between both the organs (Fig. 1).
Fig. 1.
Liver-type fatty acid-binding protein (L-FABP) mRNA transcription levels in the liver and kidney of normal dogs. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as internal control. Data are normalized to GAPDH levels. The mean L-FABP mRNA was calculated from a standard curve. Error bars represent the S.D.
Urinary L-FABP in dogs with or without renal diseases
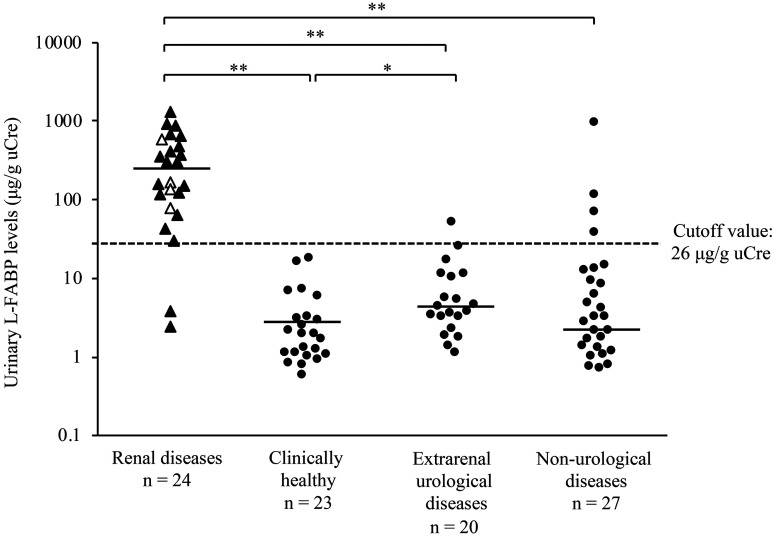
Neither gender differences nor correlation with age was observed in the urinary L-FABP concentration in clinically healthy dogs. The urinary L-FABP values of 94 dogs with or without renal diseases were assessed (Fig. 2). The urinary L-FABP was significantly higher in dogs with renal diseases than in clinically healthy dogs, those with extrarenal urological disease, and those with non-urological diseases (P<0.01). Further, the urinary L-FABP was significantly higher in dogs with extrarenal urological disease than in clinically healthy dogs (P<0.05). Based on the ROC analysis of the urinary L-FABP, the cutoff value for detecting renal disease was 26 µg/g uCre, and the sensitivity and specificity were 0.917 and 0.926, respectively. The L-FABP values of all clinically healthy dogs were below the cutoff, and those of some dogs with extrarenal urological disease and non-urological diseases were above the cutoff (n=1, dog with chronic cystitis and n=4, dogs with common bile duct obstruction, pyometra, hepatocellular carcinoma, and mandibular squamous cell carcinoma). Meanwhile, 24 dogs with renal diseases had varying urinary L-FABP values, and there were no significant differences in terms of urinary L-FABP between dogs with CKD and AKI and those with CKD at varying stages based on the IRIS classification system.
Fig. 2.
Urinary liver-type fatty acid-binding protein (L-FABP) in dogs with or without renal diseases. The triangle represents 24 dogs with renal diseases including chronic kidney disease (CKD, closed symbols, n=20) and acute kidney injury (AKI, open symbols, n=4). The circle represents 70 dogs without renal diseases including clinical healthy dogs (n=23), those with extrarenal urological disease (n=20), and those with non-urological diseases (n=27). The solid lines indicate median values (renal diseases: 238.3 µg/g uCre, clinically healthy: 1.9 µg/g uCre, extrarenal urological diseases: 4.0 µg/g uCre, and non- urological diseases: 3.2 µg/g uCre), and the dashed lines indicate the cutoff value for detecting renal diseases (26 µg/g uCre). *P<0.05 and **P<0.01.
Relationship between urinary L-FABP and renal biomarkers
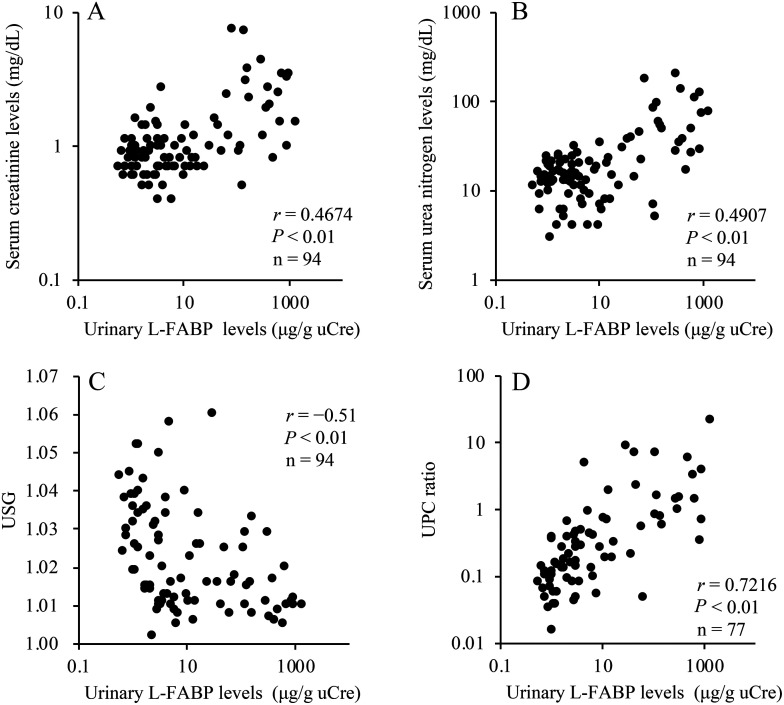
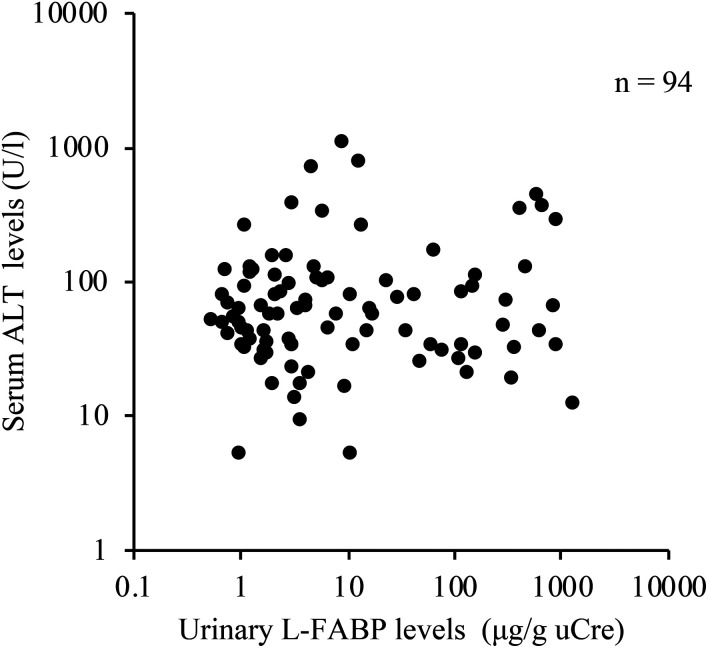
Urinary L-FABP was significantly correlated with serum creatinine level (r=0.4674, P<0.01, n=94; Fig. 3A), serum urea nitrogen (r=0.4907, P<0.01, n=94; Fig. 3B), USG (r=−0.5100, P<0.01, n=94; Fig. 3C), and UPC ratio (r=0.7216, P<0.01, n=77; Fig. 3D). There was no significant correlation between urinary L-FABP and serum ALT levels (Fig. 4).
Fig. 3.
Correlation between urinary liver-type fatty acid-binding protein (L-FABP) levels and serum creatinine (A), urea nitrogen (B), urine specific gravity (USG) (C), and urine protein/creatinine (UPC) ratio (D). The associations with urinary L-FABP were examined among serum creatinine, serum urea nitrogen and USG in 94 dogs. The relationship between urinary L-FABP and UPC ratio in 77 dogs without urinary sediment was examined.
Fig. 4.
Correlation between urinary liver-type fatty acid-binding protein (L-FABP) levels and serum alanine transaminase (ALT) levels in 94 dogs. There was no significant correlation between serum ALT and urinary L-FABP levels.
DISCUSSION
This is the first clinical report showing that dogs with renal diseases had a significantly higher urinary L-FABP than those without. The predictive cutoff value for urinary L-FABP was 26 µg/g uCre; however, all clinically healthy dogs showed an L-FABP value below the cutoff. Therefore, dogs with a high urinary L-FABP (>26 µg/g uCre) were more likely to have renal diseases. However, L-FABP values in some dogs with extrarenal urological disease and non-urological disease were above the cutoff. The two dogs with the highest urinary L-FABP values in non-urological disease (common bile duct obstruction and pyometra) were suspected to have tubular damage related to albumin released from the damaged glomerulus, because they showed elevated serum C-reactive protein and UPC levels. One dog with extrarenal urological disease and two with non-urological disease (hepatocellular carcinoma and mandibular squamous cell carcinoma) had elevated urinary L-FABP levels but did not show abnormal serum and urinary biomarker levels or abnormal kidney imaging findings. A previous study on cats demonstrated that urinary excretion of L-FABP increased before standard renal biomarkers, including serum Cre, UN, and symmetric dimethylarginine [27], whereas other studies in humans also showed that urinary excretion of L-FABP increases even before the occurrence of structural damage in the proximal tubules, due to ischemia and oxidative stress [14, 28]. A high urinary L-FABP value could be evidence of proximal tubular cells with ischemia or oxidative stress even in the absence of renal structural damage. Therefore, some dogs with a urinary L-FABP value above the cutoff might present with renal damage in the proximal tubules.
The urinary L-FABP values in dogs with renal diseases significantly varied. However, there were no significant differences in urinary L-FABP between dogs with CKD and AKI and those with CKD at varying stages based on the IRIS classification system. L-FABP is an intracellular carrier protein that is expressed in the proximal tubules of the kidney, and it reflects the extent of tubulointerstitial damage [15]. Urinary excretion of L-FABP might describe the extent of kidney damage rather than the difference in renal disease stage. However, the relationship between urinary L-FABP value and kidney damage has not been validated because this study did not conduct a histopathological analysis.
In the current study, urinary L-FABP was found to be positively correlated with serum creatinine and urea nitrogen. In dogs, serum creatinine and urea nitrogen levels were the primary diagnostic biomarkers for renal diseases such as CKD and AKI. These markers were considered as standard glomerular filtration rate (GFR) markers. Hence, they are commonly evaluated in dogs. The GFR marker values increase with decreased renal function [1, 20]. Therefore, a high urinary L-FABP value might imply a decrease in GFR. By contrast, the current study showed that urinary L-FABP had a more significant correlation with USG, which reflects tubular function, than serum GFR markers, such as creatinine and urea nitrogen. This result indicated that L-FABP, but not GFR markers, could be a useful biomarker of tubular damage. In humans, urinary L-FABP is a biomarker for the extent of tubulointerstitial damage [12, 30]. In dogs, renal proteinuria occurs primarily due to lesions in the glomerulus or, in some cases, tubular disease [21]. Moreover, persistent proteinuria may cause tubulointerstitial damage and fibrosis in the kidney [3, 4, 25]. Abnormal amounts of filtered protein accumulate in the lumen of the proximal tubular epithelial cells and contribute to renal tubulointerstitial injury via a complex cascade of intracellular events [10]. During this time, the L-FABP levels excreted in the urine may increase. The significant correlation between urinary L-FABP and UPC ratio might indicate tubular damage caused by persistent proteinuria.
In humans and mice, L-FABP was expressed in not only the kidneys but also other tissues such as those in the liver [24]. In the present study, while the urinary L-FABP levels of two dogs with common bile duct obstruction and mandibular squamous cell carcinoma were above the cutoff, those of some dogs with hepatic diseases were below the cutoff. There was no significant correlation between urinary L-FABP levels and serum ALT levels, which increase in hepatocellular injury. A previous study on humans [19] has shown that serum L-FABP is a promising biochemical marker for the early detection of hepatocellular injury. Further, in dogs, hepatocellular injury might result in increased serum L-FABP levels and urine excretion of L-FABP derived from the liver. However, our previous data showed no correlation between serum L-FABP and urinary L-FABP (Supplementary file). Considering that there was no correlation between serum ALT and urinary L-FABP, this marker was less likely to accurately reflect hepatocellular injury in dogs.
A limitation of this study was that the effect of diurnal variation on urinary L-FABP was not considered. However, in humans, diurnal variation has no effect on urinary L-FABP [7, 16], and dogs might be no exception to this. Another was the small sample size. Nevertheless, this study could provide preliminary evidence on the efficacy of urinary L-FABP values for the diagnosis of renal diseases in dogs. If proven that urinary excretion of L-FABP is more active than that of other renal markers in renal diseases such as CKD and AKI, urinary L-FABP measurement may be more clinically relevant.
The urinary L-FABP was significantly higher in dogs with renal diseases than in those without. While L-FABP was expressed in not only the kidneys but also other tissues such as those in the liver, there was no correlation between urinary L-FABP and serum ALT. Urinary L-FABP had a more significant correlation with tubular markers including USG than GFR markers such as serum creatinine and urea nitrogen. After considering the correlation between UPC ratio and urinary L-FABP, it might be useful in detecting tubular damage by caused by proteinuria. To diagnose renal diseases in dogs, urinary L-FABP measurement could be utilized in the evaluation of tubular damage.
CONFLICT OF INTEREST
Keiichi Ohata and Tsuyoshi Oikawa are the senior scientists of CMIC Holdongs Co., Ltd. (Tokyo, Japan), a company that produces ELISA kits with high sensitivity for L-FABP analysis. They were responsible for validation analyzes and measurement of an ELISA. No other potential conflicts of interest relevant to this article are reported.
Supplementary
Acknowledgments
The authors thank all the owners of the dogs included in this study. The authors also thank Dr. K. Watanabe (Joint Department of Veterinary Medicine, Faculty of Applied Biological Sciences, Gifu University) for providing us with the tissue samples.
REFERENCES
- 1.Bartges J. W.2012. Chronic kidney disease in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 42: 669–692, vi. doi: 10.1016/j.cvsm.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 2.Bass N. M., Barker M. E., Manning J. A., Jones A. L., Ockner R. K.1989. Acinar heterogeneity of fatty acid binding protein expression in the livers of male, female and clofibrate-treated rats. Hepatology 9: 12–21. doi: 10.1002/hep.1840090104 [DOI] [PubMed] [Google Scholar]
- 3.Chew D. J., DiBartola S. P., Schenck P.2011. Chapter 7: Diseases of the glomerulus. pp. 218–239. In: Canine and Feline Nephrology and Urology, 2nd ed., Elsevier, San Diego. [Google Scholar]
- 4.Erkan E., Devarajan P., Schwartz G. J.2007. Mitochondria are the major targets in albumin-induced apoptosis in proximal tubule cells. J. Am. Soc. Nephrol. 18: 1199–1208. doi: 10.1681/ASN.2006040407 [DOI] [PubMed] [Google Scholar]
- 5.Furuhashi M., Hotamisligil G. S.2008. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 7: 489–503. doi: 10.1038/nrd2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glatz J. F., van der Vusse G. J.1996. Cellular fatty acid-binding proteins: their function and physiological significance. Prog. Lipid Res. 35: 243–282. doi: 10.1016/S0163-7827(96)00006-9 [DOI] [PubMed] [Google Scholar]
- 7.Hiraki K., Kamijo-Ikemori A., Yasuda T., Hotta C., Izawa K. P., Watanabe S., Sugaya T., Kimura K.2013. Moderate-intensity single exercise session does not induce renal damage. J. Clin. Lab. Anal. 27: 177–180. doi: 10.1002/jcla.21579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichikawa D., Kamijo-Ikemori A., Sugaya T., Yasuda T., Hoshino S., Igarashi-Migitaka J., Hirata K., Kimura K.2012. Renal liver-type fatty acid binding protein attenuates angiotensin II-induced renal injury. Hypertension 60: 973–980. doi: 10.1161/HYPERTENSIONAHA.112.199828 [DOI] [PubMed] [Google Scholar]
- 9.Ichikawa D., Kamijo-Ikemori A., Sugaya T., Shibagaki Y., Yasuda T., Hoshino S., Katayama K., Igarashi-Migitaka J., Hirata K., Kimura K.2015. Human liver-type fatty acid-binding protein protects against tubulointerstitial injury in aldosterone-induced renal injury. Am. J. Physiol. Renal Physiol. 308: F114–F121. doi: 10.1152/ajprenal.00469.2014 [DOI] [PubMed] [Google Scholar]
- 10.Jacob F., Polzin D. J., Osborne C. A., Neaton J. D., Kirk C. A., Allen T. A., Swanson L. L.2005. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J. Am. Vet. Med. Assoc. 226: 393–400. doi: 10.2460/javma.2005.226.393 [DOI] [PubMed] [Google Scholar]
- 11.Kabir M., Catalano K. J., Ananthnarayan S., Kim S. P., Van Citters G. W., Dea M. K., Bergman R. N.2005. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am. J. Physiol. Endocrinol. Metab. 288: E454–E461. doi: 10.1152/ajpendo.00203.2004 [DOI] [PubMed] [Google Scholar]
- 12.Kamijo A., Sugaya T., Hikawa A., Okada M., Okumura F., Yamanouchi M., Honda A., Okabe M., Fujino T., Hirata Y., Omata M., Kaneko R., Fujii H., Fukamizu A., Kimura K.2004. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am. J. Pathol. 165: 1243–1255. doi: 10.1016/S0002-9440(10)63384-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamijo A., Kimura K., Sugaya T., Yamanouchi M., Hikawa A., Hirano N., Hirata Y., Goto A., Omata M.2004. Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J. Lab. Clin. Med. 143: 23–30. doi: 10.1016/j.lab.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 14.Kamijo-Ikemori A., Sugaya T., Kimura K.2006. Urinary fatty acid binding protein in renal disease. Clin. Chim. Acta 374: 1–7. doi: 10.1016/j.cca.2006.05.038 [DOI] [PubMed] [Google Scholar]
- 15.Kamijo-Ikemori A., Sugaya T., Matsui K., Yokoyama T., Kimura K.2011. Roles of human liver type fatty acid binding protein in kidney disease clarified using hL-FABP chromosomal transgenic mice. Nephrology (Carlton) 16: 539–544. doi: 10.1111/j.1440-1797.2011.01469.x [DOI] [PubMed] [Google Scholar]
- 16.Kamijo-Ikemori A., Sugaya T., Ichikawa D., Hoshino S., Matsui K., Yokoyama T., Yasuda T., Hirata K., Kimura K.2013. Urinary liver type fatty acid binding protein in diabetic nephropathy. Clin. Chim. Acta 424: 104–108. doi: 10.1016/j.cca.2013.05.020 [DOI] [PubMed] [Google Scholar]
- 17.Maatman R. G., van de Westerlo E. M., van Kuppevelt T. H., Veerkamp J. H.1992. Molecular identification of the liver- and the heart-type fatty acid-binding proteins in human and rat kidney. Use of the reverse transcriptase polymerase chain reaction. Biochem. J. 288: 285–290. doi: 10.1042/bj2880285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okazaki M., Oikawa T., Sugaya T.2015. The biomarker for CKD: urinary L-FABP - from molecular function to clinical significance. Nippon Yakurigaku Zasshi 146: 27–32. doi: 10.1254/fpj.146.27 [DOI] [PubMed] [Google Scholar]
- 19.Pelsers M. M. A. L., Morovat A., Alexander G. J. M., Hermens W. T., Trull A. K., Glatz J. F. C.2002. Liver fatty acid-binding protein as a sensitive serum marker of acute hepatocellular damage in liver transplant recipients. Clin. Chem. 48: 2055–2057. doi: 10.1093/clinchem/48.11.2055 [DOI] [PubMed] [Google Scholar]
- 20.Polzin D. J.2011. Chronic kidney disease in small animals. Vet. Clin. North Am. Small Anim. Pract. 41: 15–30. doi: 10.1016/j.cvsm.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 21.Roura X., Elliott J., Grauer G. F.2017. Proteinuria. pp. 50–59. In: BSAVA Manual of Canine and Feline Nephrology and Urology, 3rd ed. (Elliott, J., Grauer, G. and Westropp, J. L. eds.), British Small Animal Veterinary Association, Gloucester. [Google Scholar]
- 22.Sato R., Soeta S., Miyazaki M., Syuto B., Sato J., Miyake Y., Yasuda J., Okada K., Naito Y.2002. Clinical availability of urinary N-acetyl-beta-D-glucosaminidase index in dogs with urinary diseases. J. Vet. Med. Sci. 64: 361–365. doi: 10.1292/jvms.64.361 [DOI] [PubMed] [Google Scholar]
- 23.Sasaki A., Sasaki Y., Iwama R., Shimamura S., Yabe K., Takasuna K., Ichijo T., Furuhama K., Satoh H.2014. Comparison of renal biomarkers with glomerular filtration rate in susceptibility to the detection of gentamicin-induced acute kidney injury in dogs. J. Comp. Pathol. 151: 264–270. doi: 10.1016/j.jcpa.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 24.Su A. I., Wiltshire T., Batalov S., Lapp H., Ching K. A., Block D., Zhang J., Soden R., Hayakawa M., Kreiman G., Cooke M. P., Walker J. R., Hogenesch J. B.2004. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA 101: 6062–6067. doi: 10.1073/pnas.0400782101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang S., Sheerin N. S., Zhou W., Brown Z., Sacks S. H.1999. Apical proteins stimulate complement synthesis by cultured human proximal tubular epithelial cells. J. Am. Soc. Nephrol. 10: 69–76. doi: 10.1681/ASN.V10169 [DOI] [PubMed] [Google Scholar]
- 26.Veerkamp J. H., Zimmerman A. W.2001. Fatty acid-binding proteins of nervous tissue. J. Mol. Neurosci. 16: 133–142, discussion 151–157. doi: 10.1385/JMN:16:2-3:133 [DOI] [PubMed] [Google Scholar]
- 27.Watanabe A., Ohata K., Oikawa T., Sugaya T., Miyazaki M., Satoh H., Katayama M.2021. Preliminary study of urinary excretion of liver-type fatty acid-binding protein in a cat model of chronic kidney disease. Can. J. Vet. Res. 85: 156–160. [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y., Xie Y., Shao X., Ni Z., Mou S.2015. L-FABP: a novel biomarker of kidney disease. Clin. Chim. Acta 445: 85–90. doi: 10.1016/j.cca.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto T., Noiri E., Ono Y., Doi K., Negishi K., Kamijo A., Kimura K., Fujita T., Kinukawa T., Taniguchi H., Nakamura K., Goto M., Shinozaki N., Ohshima S., Sugaya T.2007. Renal L-type fatty acid--binding protein in acute ischemic injury. J. Am. Soc. Nephrol. 18: 2894–2902. doi: 10.1681/ASN.2007010097 [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama T., Kamijo-Ikemori A., Sugaya T., Hoshino S., Yasuda T., Kimura K.2009. Urinary excretion of liver type fatty acid binding protein accurately reflects the degree of tubulointerstitial damage. Am. J. Pathol. 174: 2096–2106. doi: 10.2353/ajpath.2009.080780 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.