Figure 6.
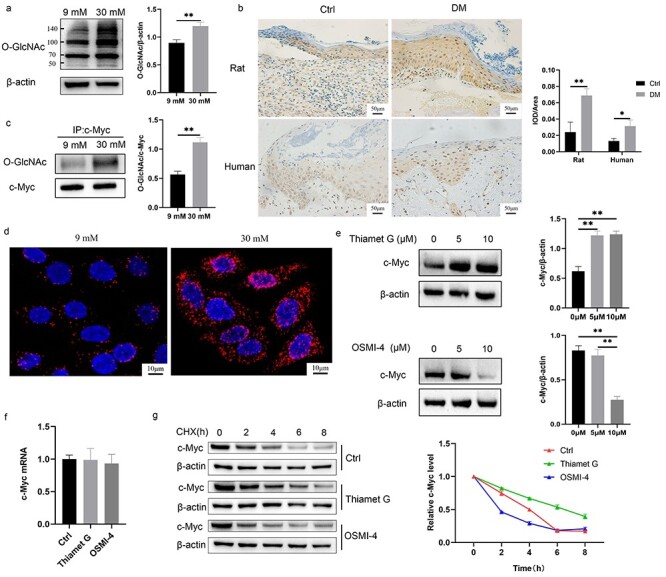
O-GlcNAcylation of c-Myc made c-Myc proteins more stable. (a, b) HaCaT cells (a) cultured with 30 mM glucose and keratinocytes (b) at the diabetic wound margin showed higher level of O-GlcNAc. (c, d) Increased O-GlcNAcylation of c-Myc with 30 mM glucose was confirmed using immunoprecipitation (c) and proximity ligation assay (d). (e, f) The protein (e) and mRNA (f) expression of c-Myc was detected after treatment with 10 μM Thiamet G or OSMI-4 for 24 h. (g) c-Myc protein stability was measured using CHX (a protein synthesis inhibitor). Cells were pre-incubated for 24 h with 10 μM Thiamet G or OSMI-4 and subsequently treated with 50 μM CHX for different times (0, 2, 4, 6, 8 h) before harvesting. Compared with the Ctrl group, Thiamet G delayed the degradation rate of c-Myc protein, while OSMI-4 accelerated the degradation rate of c-Myc protein. Scale bar: 50 μm, 10 μm. *p < 0.05, **p < 0.01. CHX cycloheximide, Ctrl control, DM diabetes mellitus, IOD integrated optical density, O-GlcNAc O-linked N-acetylglucosamine
