Abstract:
A 1-year old male patient with Williams syndrome and multiple prior interventions presented for surgical repair of his descending aorta (DA) through a left thoracotomy. Concerns for significant bleeding and spinal cord protection led the care team to consider a left heart bypass (LHB) circuit with options for pump sucker use, heat exchange capacity, and the possibility of converting to traditional cardiopulmonary bypass (CPB). A traditional CPB circuit with a roller-head arterial pump was assembled with a bypass line around the cardiotomy venous reservoir (CVR). Excluding the CVR with this line allowed for a closed LHB circuit. A second pump head was integrated to both recirculate the CVR volume and to serve as a means for controlled volume administration to the closed LHB circuit. Pump sucker return directed to the CVR could easily be transfused back to the patient. The patient was placed on the hybrid LHB circuit and cooled to 32°C. DA clamps were placed. Upper body dynamic blood pressure was managed for a target mean of 50 mmHg, the left atrial pressure (LAP) was maintained in the 5–7 mmHg range, and the nonpulsatile lower body blood pressure was targeted at 40–50 mmHg. Cerebral near-infrared spectroscopy (NIRS) helped guide volume and pressure management. The surgeons placed two long-segment patches on the DA, moving clamps as needed. The patient was rewarmed and separated from the hybrid LHB circuit after 82 minutes. Closed circuit LHB can be provided with a roller-head hybrid circuit incorporating an oxygenator for gas exchange, central cooling and warming, and arterial line filtration along with a CVR for pump sucker use and controlled transfusion to the patient.
Keywords: left heart bypass, hybrid left heart bypass circuit, pump suckers, METEOR circuit, pediatric, Williams syndrome
Surgery involving the descending aorta (DA) carries the risk of spinal cord deficit and paraplegia and there is no consensus on the preferred approach to mitigate risk. Some surgeons prefer the “clamp-and-sew” technique, especially in noncomplicated surgeries with a short projected cross clamp time. A single center retrospective study found no difference in complications, including paraplegia, when left heart bypass (LHB) was used vs. none in adults with descending thoracic aortic aneurysm undergoing surgical repair (1). In more complex cases, such as cases with recurrent coarctation or those without sufficient collateral flow beyond the lesion, actively perfusing the lower body is preferred. The use of LHB is just one option. Full cardiopulmonary bypass (CPB) with deep hypothermia and circulatory arrest may also be used with comparable outcomes relative to LHB (2–4). Another approach that has been described is the interposition of an ascending-to-descending aortic bypass graft (5).
LHB for the repair of thoracoabdominal lesions without sufficient collateral circulation typically involves a simple circuit consisting of inflow and outflow lines, a centrifugal head, pressure monitoring, and a bubble detector (6,7). These circuits have been in use since the 1960s (8). Such a simple circuit is enough to provide LHB because only a shunt around the lesion is required. Patient pulmonary function is maintained for oxygenation and ventilation while the circuit draws enough blood from the left atrium to allow for both adequate native heart ejection to the upper body and nonpulsatile centrifugal pump perfusion to the lower body. Clinical care teams have used circuits ranging from the simple circuits mentioned earlier with limited anticoagulation to traditional CPB circuits with full anticoagulation and deep hypothermia (2,3). This range of circuits has been uses to address concerns for neurologic, renal, and spinal cord protection for a variety of aortic lesions. Our institution uses conventional LHB for patients with coarctation of the aorta with insufficient collateral circulation. Our circuit typically consists of a centrifugal pump with inflow and outflow lines and pressure monitoring. Full heparinization is used with a target activated clotting time (ACT) ≥400 seconds (9). As subclinical clotting can occur at lower levels of anticoagulation, even with coated circuits, we have not transitioned to lower levels of heparinization (10). Having a patient with Williams syndrome needing long segment DA reconstruction had us consider additional LHB circuit options. The planned approach was through a left thoracotomy and there were concerns for neurologic, renal and spinal cord protection along with risks for bleeding. We devised a hybrid LHB circuit to address the needs of this individual patient extrapolating from prior experience with a similar hybrid circuit used in a different setting. In 2016, we devised a closed circuit on our heart-lung machine to serve as extracorporeal membrane oxygenation (ECMO) for part of the case and then as a traditional CPB circuit for another part (11). This circuit allowed us to place a fetus on ECMO through cannulation of the neck vessels before separation from the placenta. After birth, the patient was transferred to a separate operating room where surgery could be performed using the traditional CPB circuit by changing the flow path through the custom circuit.
Traditional LHB has the advantage of being a closed system. This allows shunting of volume from the left atrium, bypassing a DA lesion, which is surgically isolated, to the distal aorta or femoral artery. Patient management during this process involves maintaining a positive left atrial pressure (LAP) while shunting blood to the distal aorta. The intravascular volume does not change when the patient is placed on LHB, but it is distributed between two parallel circulations: the upper body circulation that is dependent on preload and native cardiac function, and the lower body circulation that is dependent on bypass flow support. On the other end of the circuit spectrum is traditional CPB which is most often an open system where the patient’s intravascular volume can be manipulated since the venous reservoir is open to atmosphere and has capacitance. Traditional CPB includes an oxygenator, heat exchanger, and on some models, an integrated arterial line filter (ALF). A cardiotomy venous reservoir (CVR) processes both venous return and pump sucker return. Changes to the venous limb of a CPB circuit, either with a traditional tubing clamp or with an electronic occluder, impacts venous drainage and thus intravascular volume. Traditional CPB typically transfers a portion of the intravascular volume to the CVR when the surgeon asks to “empty the patient out.” This is in contrast to the LHB circuit that does not normally have the ability to change intravascular volume.
For the safe conduct of this patient’s surgery, we needed a LHB circuit but also some of the features offered by a traditional CPB circuit, namely, the ability to centrally cool and warm, to recover shed blood with field suckers, and the inclusion of in-line blood gas and hematocrit monitoring. The inclusion of gas exchange and arterial line filtration with the oxygenator were additionally beneficial. Incorporation of a mini pump allowed for CVR volume recirculation and controlled transfusion of this volume into the closed circuit as needed. The circuit for our patient provided a closed LHB circuit with the functionality of pump sucker use, central cooling and heating, oxygenator functions with real-time blood gas monitoring, arterial line filtration, the ability for controlled transfusion from the CVR, and the capability to transition to full CPB if needed.
DESCRIPTION
Patient History
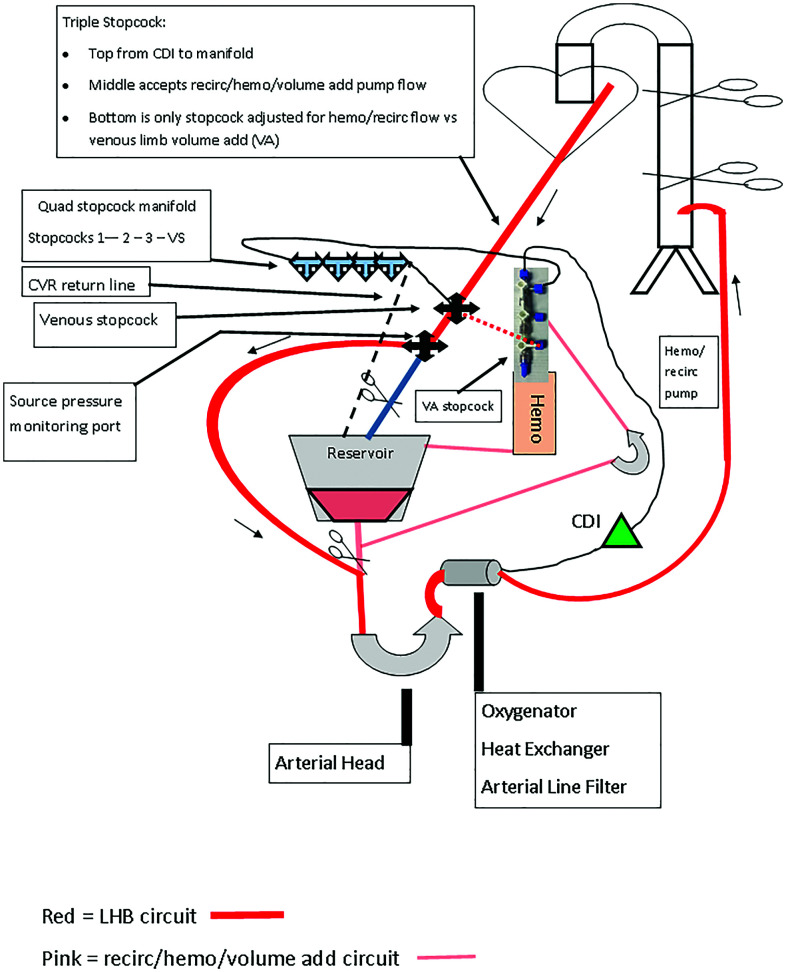
Our patient was a 1-year old, 8 kg (BSA 0.4 m2) male with Williams syndrome who initially presented with supra-aortic stenosis, a hypoplastic aortic arch and hypoplasia of the main and branch pulmonary arteries. At 4 months of age, he underwent patch reconstruction of the sino-tubular junction, aortic arch, and repair of the pulmonary arteries from hilum to hilum. At 10 months of age, the patient underwent cardiac catheterization for placement of a Formula stent (Cook Medical, Limerick, Ireland) in the proximal DA for coarctation at and beyond the aortic arch patch. At 1 year of age, he returned to the catheterization lab for recurrent coarctation within and beyond the stent. Significant right and left coronary artery obstructions were found. Distal and main pulmonary arteries were narrowed. There were bilateral renal artery stenoses. The descending and abdominal aorta were diffusely small. The distal pulmonary arteries were balloon dilated and temporizing balloon angioplasty was performed on the recurrent coarctation. Two weeks later, the patient underwent cardiac surgery on CPB through median sternotomy to address the coronary obstructions and the pulmonary artery stenoses by patch plasties. The transverse arch and proximal DA were also dissected out to prepare the patient for a planned extensive descending aortoplasty through a left thoracotomy. Three days after the median sternotomy, the patient was brought back to the operating room for the second stage of his repair: total DA patch plasty through a left thoracotomy. Because of the extent of DA reconstruction required, the decision was made to use a hybrid LHB circuit as depicted in Figure 1.
Figure 1.
Hybrid left heart bypass circuit with integrated oxygenator, reservoir, and accessory head for recirculation and volume administration.
Operative Course
Following his cardiac surgery 3 days prior to the current presentation, the patient remained in the cardiac intensive care unit while awaiting repair of recurrent coarctation and a diffusely small DA. His trachea remained intubated with a 3.5-mm cuffed endotracheal tube (ETT) and the invasive monitoring lines used for the previous surgery were kept in situ including a central venous catheter in the right internal jugular vein, a left radial arterial catheter, and an LAP line. He was sedated with a continuous infusion of morphine and dexmedetomidine and was hemodynamically stable, with no inotropic support. In the operating room, he received additional intravenous sedatives and muscle relaxants and was maintained on isoflurane. Additional sites for invasive arterial blood pressure monitoring were added for this procedure in the right axillary artery and the right femoral artery. Bilateral cerebral oximetry sensors were placed. After being positioned in the right lateral decubitus position, and to facilitate surgical exposure during thoracotomy, lung isolation was performed by pushing the existing ETT into the right mainstem bronchus. The adequacy of single right lung ventilation was verified by auscultation and by visualizing the tip of the ETT in the right main bronchus using a C-MAC fiberoptic video scope (Karl Storz SE and Company, Tuttlingen, Germany).
The hybrid LHB circuit consisted of a Terumo CAPIOX FX05 oxygenator with hardshell reservoir (Terumo Cardiovascular, Inc., Ann Arbor, MI), a custom tubing pack with a 3/16” arterial line and ¼” venous, boot and CVR shunt lines (Sorin Group USA, Arvada, CO). These components were assembled on a Stockert S5 heart-lung machine (LivaNova PLC, London, UK). The circuit was CO2 flushed before being crystalloid primed with heparinized Plasma Lyte-A 7.4 (Baxter Healthcare, Deerfield, IL) and recirculated through a 5-μm prebypass filter. The circuit was then blood primed using heparinized red blood cells premixed in the blood bank with plasma. Prebypass ultrafiltration with additional Plasma-Lyte A and 0.45% normal saline was performed. The final blood-primed circuit consisted of approximately 200 mL in the closed portion of the circuit and an additional 100 mL in the reservoir and recirculation circuit. Prime medications included 900 units heparin (3 units heparin/mL of prime), 10 mEq sodium bicarbonate, and 900 mg calcium gluconate. A lab sample of the circuit prime revealed normal pH, blood gas, and electrolyte values. To note, the ionized calcium level was 1.4 mmol/L in the prime approximating the prebypass patient value of 1.3 mmol/L. This helped mitigate any changes in myocardial function upon initiating LHB. The circuit prime was maintained at 34°C.
The patient was anticoagulated with 350 u/kg of heparin sodium (Mylan Pharmaceuticals, Canonsburg, PA). The distal DA was cannulated at the diaphragm with an 8 Fr Biomedicus arterial cannula rated to 0.7 LPM in the inflow position. The left atrial appendage was cannulated with a 14 Fr DLP bullet-tip venous cannula rated to 0.8 LPM as a sourcing cannula (Medtronic, Minneapolis, MN). These cannulae were deemed sufficient to handle the anticipated LHB flows but not full CPB. An ACT ≥ 400 seconds was verified on a Hemochron Signature Elite point of care device (Accriva Diagnostics, San Diego, CA). Pump suckers were turned on. LHB using the shunt around the CVR was instituted and antibiotic coverage with 200 mg Cefazolin (25 mg/kg) was added to the circuit. The patient was cooled to 32°C. Perfusionist interventions, in addition to traditional LHB management, are listed in Table 1.
Table 1.
Action items with process steps.
| Action | Process |
|---|---|
| Volume add (VA) | 1. Turn “VA” stopcock to direct recirc pump flow to the venous limb for effect |
| Volume subtract (VS) | 1. Turn “VS” stopcock to direct CDI/manifold flow to the CVR for effect |
| Arterial blood gas | 1. Turn “VS” stopcock to direct CDI/manifold flow to the CVR |
| 2. Flush hub into flow path and then remove arterial sample | |
| 3. Turn “VS” stopcock to direct CDI/manifold flow to the venous limb | |
| 4. Give volume as necessary to account for loss during sampling | |
| Venous blood gas | 1. Turn stopcock “2” to stop flow through manifold |
| 2. Use stopcock “3” to pull back blood from venous limb and flush this into the syringe on stopcock “2” | |
| 3. Use same process to obtain venous limb sample | |
| 4. Deair manifold to CVR and then turn stopcocks to allow for flow to the venous limb | |
| 5. Give volume as necessary to account for loss during sampling | |
| Administer medication | 1. Connect medication syringe to stopcock “2” |
| 2. Deair hub and administer to venous limb |
The DA was mobilized in its entirety. The surgeons placed clamps to isolate the proximal half of the DA. Approximately 100 mL of circulating volume was removed using the volume subtract (VS) stopcock to best balance the upper and lower body pressures. A pulmonary homograft patch was used to enlarge the DA. This included cutting the existing stent. The distal half of the DA was perfused. All intercostals were preserved. The target nonpulsatile distal perfusion pressure was 40–50 mmHg with an LAP 5–7 mmHg. The target dynamic upper body pressure was 65–75 systolic and 40–50 diastolic. The patient’s circulation was supported by titrating dopamine and norepinephrine infusions as needed, in addition to using the volume add (VA) feature to return shed blood. Continuous direct communication between the anesthesiologist and perfusionist was used for decision-making regarding maintenance of the blood pressure targets and blood gas values for the upper body vs. the pump circuit for the lower body. Bilateral near-infrared spectroscopy (NIRS) values were kept within 10% of baseline. An in-line blood gas monitor (Terumo Cardiovascular, Inc., Ann Arbor, MI) followed circuit (lower body) arterial blood gases along with venous saturation and hematocrit values in real time.
Once the first patch was completed, the surgeons repositioned the aortic clamp to isolate the distal half of the DA, while perfusing the upper half. Bleeding occurred after release of the clamps and sutures were placed as necessary. Pump sucker blood was processed in the CVR and returned to the closed LHB circuit as needed using the accessory pump directing volume to the source limb of the circuit via the VA stopcock. The completed DA reconstruction included two patches that extended from the left subclavian artery to the diaphragm (8.5 cm). The patient was rewarmed to 34–35°C and separated from LHB after 82 minutes. Heparinization was reversed with 4 mg/kg of protamine. Bleeding sites were controlled surgically along with the addition of platelets titrated to effect by clinical judgment for a total of 150 mL. Residual pump circuit volume was run through a Sorin Xtra cell saver device (Sorin Group USA, Arvada, CO) and this volume was transfused to the patient. The left lung was fully reinflated under direct vision by retrieving the ETT back to its normal position above the carina. An arterial blood gas was obtained and confirmed adequate gas exchange. The incision was closed and the patient was transferred to the cardiac intensive care unit. On postoperative day (POD) 1, his sedation was lightened to allow for observation of movement. His trachea was extubated on POD 3 without complications. He was by then moving vigorously and did not have any evidence of neurologic or renal injury. A transthoracic echocardiogram performed on POD 11 was reassuring and revealed an unobstructed aortic arch and descending thoracic aorta with normal flow extending into the abdominal aorta. The patient was discharged home on POD 17.
Comments
LHB is commonly considered a rather simple therapy for perfusionists to provide. The most basic circuits include minimal tubing and a centrifugal pump head with limited anticoagulation required. More involved circuits including those with a standalone oxygenator and/or standalone heat exchanger, along with full traditional CPB circuits, have been described for similar surgeries (2–4,12–15). Brindisi et al. described a multi-modal system that could flex for various closed circuit applications including LHB (12). Leach et al. described an adult LHB circuit inclusive of an oxygenator, which is important if surgery requires single lung ventilation or if there is pulmonary dysfunction (13). Bisleri et al. described a novel setup for their adult population, which gave them the ability to transition between LHB with or without oxygenator support and traditional CPB depending on surgical case progress (14). Other authors, including Koukouchos and Rokkas, describe the other end of the circuit spectrum with use of traditional CPB circuits for complex operations on the thoracoabdominal aorta, allowing for moderate to deep hypothermia, which may significantly mitigate spinal cord injury in adults (15). These authors devised unique solutions for their primarily adult population patients. The lack of available standalone bypass circuit heat exchangers is a recent development forcing clinicians to consider inclusion of an oxygenator with its integral heat exchanger if central temperature management is desired (16). One safety item not commonly mentioned in case reports and series is the use of arterial line filtration. While LHB, when serving as a simple shunt, may not need this additional safety measure, it should become a consideration when a heat exchanger, standalone oxygenator, and pump suckers are incorporated. The vast majority of pediatric centers around the world report that they use ALFs for traditional CPB (17). As LHB circuits merge with traditional CPB circuits, the ALF must be a consideration since it has been well established that bypass circuits are imperfect in removing gaseous microemboli (18,19). The advent of oxygenator-heat exchangers with integrated arterial filters makes this point nearly moot since the addition of just the “oxygenator” includes such features. Additionally, “oxygenators” come with an attached CVR that simplifies one’s setup, including limiting overall prime volume.
Repair of discrete coarctations of the aorta commonly include conventional LHB circuit when insufficient collateral circulation exists but the current patient’s presentation was quite different and therefore required additional considerations. Our hybrid LHB circuit was designed for an 8-kg patient with Williams syndrome presenting with recurrent obstruction and a diffusely small DA requiring extensive reconstruction. Pump sucker use and heat exchange were desired. Our program has successfully used full heparinization for conventional LHB cases so it was not as much of a stretch to change to a hybrid circuit with pump suckers since we already used full-dose anticoagulation. The Terumo CAPIOX FX-05’s inclusion of an integrated arterial filter was an additional safeguard and unlike the other reports previously mentioned. Since the oxygenator is integral to the circuit 100% of the time, so is this safety measure. We believe this is important since LHB can be considered a form of assisted venous drainage (albeit from an arterial source in this case), which has been implicated for increased risk of GME (18). That is rarely mentioned in the literature but should not be overlooked if one is interested in limiting GME transmission to their patients.
Another consideration is that our hybrid LHB circuit setup could easily be configured with a centrifugal arterial pump. Regardless of the arterial pump type, we recommend monitoring the venous line pressure and servoregulating this to the arterial pump to help prevent entrainment or cavitation of air if the source limb or cannula becomes obstructed in any way. Of note, recognized air entrainment into the closed LHB circuit could be managed by clamping the circuit so that the blood flow path went through the CVR, with partial occlusion of the venous limb, to maintain intravascular volume while remedying the source of air entrainment. This is important since venous limb air results in increased arterial limb GME even with an ALF (19). Additionally, we monitored the inflow and outflow limbs with ultrasonic flow probes which could give an early indication of flow fluctuations but not necessarily acute obstructions. Servoregulated pressure monitoring would be the preferred safety measure for this concern.
It is worth noting that one may elect to simply use a standard CPB circuit for LHB cases instead of creating a hybrid circuit like the one we chose for this patient. The main drawback we found with that option is that, in addition to balancing the upper and lower body pressures with the shunted flow, the perfusionist must continually assess source flow and how much to occlude the source (“venous”) limb. This is analogous to being in a constant state of weaning from CPB. While those combined tasks may be manageable in a stable setting, we believe that the cognitive workload is more manageable with a closed circuit when bleeding becomes an issue. Blood can be precisely returned to the patient from the CVR at a designated flow rate using the VA stopcock when intravascular volume changes are more dynamic with bleeding. That portion of the circuit also included a hemoconcentrator which we used for prebypass ultrafiltration. We kept that in the final circuit in anticipation of bleeding which could temporarily shunt volume to the pump suckers requiring crystalloid volume administration to the patient to maintain circulating volume. We could then ultrafilter the crystalloid volume off when there was no longer hold-up in the suckers. Fortunately, this was not an issue for the present patient but we believe it is an important clinical consideration. The VS feature was also useful during initiation of LHB when the DA clamp was placed and it was advantageous to decrease the circulating volume. A final comment regarding this circuit is that the option for full cardiopulmonary support is easily achieved with changing tubing clamp locations on the pump circuit along with changing cannulation sites at the surgical field. The hybrid LHB circuit we used for this Williams syndrome patient provided us all of the functionality the care team desired in a manageable platform for the perfusionist. We believe that this hybrid LHB circuit is advantageous when central cooling and warming, plus utilization of pump suckers, are desired for select patients requiring LHB for thoracoabdominal lesions.
REFERENCES
- 1.Coselli JS. The use of left heart bypass in the repair of thoracoabdominal aortic aneurysms: current techniques and results. Semin Thorac Cardiovasc Surg. 2003;15:326–32. [DOI] [PubMed] [Google Scholar]
- 2.Kouchoukos NT, Wareing TH, Izumoto H, et al. . Elective hypothermia and cardiopulmonary bypass and circulatory arrest for spinal cord protection during operations on the thoracoabdominal aorta. J Thorac Cardiovasc Surg. 1990;99:659–64. [PubMed] [Google Scholar]
- 3.Wahlgren CM, Blohmé L, Günther A, et al. . Outcomes of left heart bypass versus circulatory arrest in elective open surgical descending and thoraco-abdominal aortic repair. Eur J Vasc Endovasc Surg. 2017;53:672–8. [DOI] [PubMed] [Google Scholar]
- 4.Papadimas E, Tan YK, Qi Q, et al. . Left heart bypass versus circulatory arrest for open repair of thoracoabdominal aortic pathologies. ANZ J Surg. 2020;90:2434–40. [DOI] [PubMed] [Google Scholar]
- 5.Said SM, Burkhart HM, Dearani JA, et al. . Ascending-to-descending aortic bypass: a simple solution to a complex problem. Ann Thorac Surg. 2014;97:2041–8. [DOI] [PubMed] [Google Scholar]
- 6.Martin TD. Thoracic aortic surgery and cardiopulmonary bypass. In: Mora CT, ed. Cardiopulmonary Bypass: Principles and Techniques of Extracorporeal Circulation. New York, NY: Springer-Verlag; 1995:329–39. [Google Scholar]
- 7.Mongero LB, Beck JR. eds. On Bypass: Advanced Perfusion Techniques. Totowa: Humana Press Inc.; 2008:317–9. [Google Scholar]
- 8.Baird RJ, Labrosse CJ, Lajos TZ.. Survey of mechanical assistance of the circulation and the present status of left-heart bypass. Can Med Assoc J. 1966;95:646–51. [PMC free article] [PubMed] [Google Scholar]
- 9.Matte GS, Howe RJ, Ibla J, Emani S, et al. . Transition from Hemochron Response to Hemochron Signature Elite activated clotting time devices in a congenital cardiac surgery practice. J Extra Corpor Technol. 2019;51:221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorman RC, Ziats N, Rao AK, et al. . Surface-bound heparin fails to reduce thrombin formation during clinical cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1996;111:1–11. [DOI] [PubMed] [Google Scholar]
- 11.Matte GS, Connor KR, Toutenel NA, et al. . A modified EXIT-to-ECMO with optional reservoir circuit for use during an EXIT procedure requiring thoracic surgery. J Extra Corpor Technol. 2016;48:35–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Brindisi N, Stammers A, Trowbridge C, et al. . Use of a multi-modality life support system. J Extra Corpor Technol. 2008;40:268–70. [PMC free article] [PubMed] [Google Scholar]
- 13.Leach WR, Sundt TM, Moon MR, et al. . Oxygenator support for partial left-heart bypass. Ann Thorac Surg. 2001;72:1770–1. [DOI] [PubMed] [Google Scholar]
- 14.Bisleri G, Tisi G, Negri A, et al. . The bicircuit system: Innovative perfusional options for surgical treatment of the thoracic aorta. Ann Thorac Surg. 2005;79:678–81. [DOI] [PubMed] [Google Scholar]
- 15.Kouchoukos NT, Rokkas CK.. Hypothermic cardiopulmonary bypass for spinal cord protection: rationale and clinical results. Ann Thorac Surg. 1999;67:1940–2. [DOI] [PubMed] [Google Scholar]
- 16.Medtronic Customer Communication . Discontinuation of BIOtherm™ and ECMOtherm™ Heat Exchangers. Document UC202010572 EN. Minneapolis, MN: Medtronic Inc. January 2020.
- 17.Harvey BH, Shann KG, Fitzgerald D, et al. . International Pediatric Perfusion Practice: 2011 Survey Results. J Extra Corpor Technol. 2012;44:186–93. [PMC free article] [PubMed] [Google Scholar]
- 18.Win KN, Wang S, Undar A.. Microemboli generation, detection and characterization during CPB procedures in neonates, infants, and small children. ASAIO J. 2008;54:486–90. [DOI] [PubMed] [Google Scholar]
- 19.Matte GS, Connor KR, Liu H, et al. . Arterial limb microemboli during cardiopulmonary bypass: Observations from a congenital cardiac surgery practice. J Extra Corpor Technol. 2016;48:5–10. [PMC free article] [PubMed] [Google Scholar]