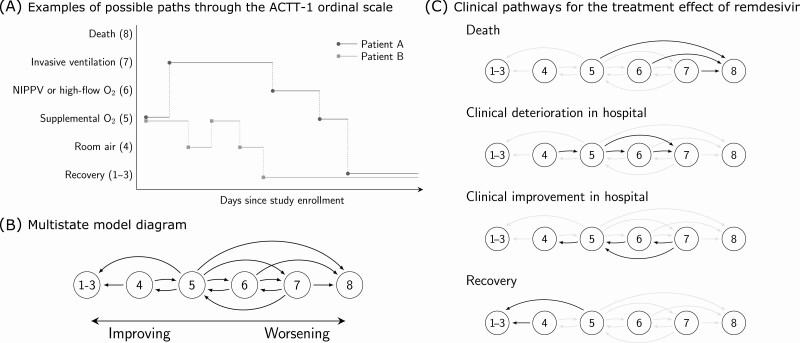
Figure 1.
Multistate model for clinical progression for patients enrolled in the Adaptive COVID-19 Treatment Trial-1 (ACTT-1). A, Examples of possible paths through the ACTT-1 ordinal score (OS) scale. Both patients A and B are on supplemental oxygen (OS 5) at baseline. A standard time-to-event analysis assesses whether treatment with remdesivir shortens the expected time until the patients enter the recovered state (OS 1–3). Multistate analysis assesses whether treatment with remdesivir alters the dynamics of how patients travel throughout the ordinal scale over the course of the study. B, Multistate model diagram. Patients transition between states continuously in time. Arrows indicate which direct transitions are possible. For example, a patient starting on room air may transition to discharge or supplemental oxygen. However, the model assumes that a patient on room air would not be intubated without first receiving supplemental oxygen, whether “observed” or not from the perspective of data capturing. Note that the data are daily snapshots of each patient’s status and that multiple transitions are possible within the same day. C, Clinical pathways for the treatment effect of remdesivir. Hazard ratio for remdesivir versus placebo is assumed to be common to all transitions within each transition group. For instance, we estimate that remdesivir slows down the rate of clinical deterioration within the hospital by a relative 26% (95% CI: 6%–43%) and that this effect applies to worsening from room air to supplemental oxygen (OS 4–5), supplemental oxygen to noninvasive positive-pressure ventilation (OS 5–6), or supplemental oxygen to invasive ventilation (OS 5–7). Sensitivity to groupings of transitions is explored in the supplement and the results are shown to be robust to how transitions are grouped. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; NIPPV, noninvasive positive-pressure ventilation.