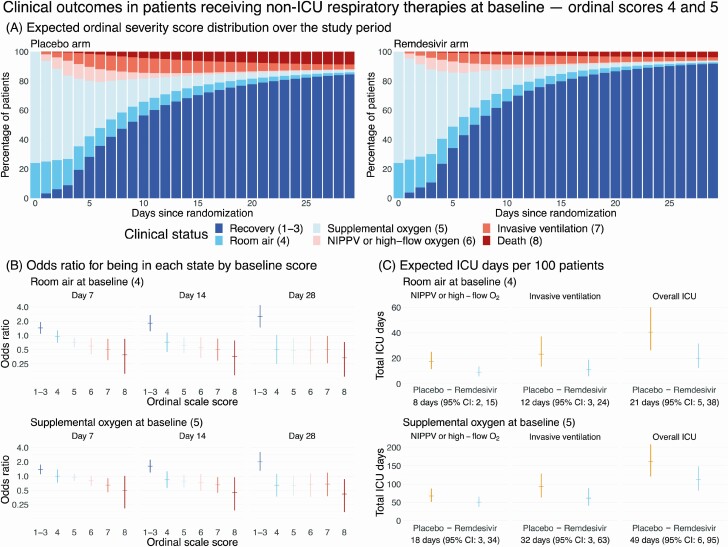
Figure 3.
Clinical impact of remdesivir in patients receiving non-ICU respiratory therapies at baseline ordinal scores 4 and 5. A, Stacked probability plots showing the expected distribution of ordinal severity scores by treatment arm at each day post-randomization among patients receiving nonintensive therapies outside the ICU setting at baseline, assuming the initial distribution of baseline ordinal score 4 and 5 patients is the same as was observed in the Adaptive Clinical Treatment Trial-1. B, Ratio of odds that a patient on room air (top row) or supplemental oxygen (bottom row) at baseline will be in each ordinal score on remdesivir versus placebo at days 7, 14, and 28 post-randomization. Each odds ratio is shown with a 95% bootstrap CI. C, Expected number of ICU days per 100 patients by baseline ordinal score. Each plot shows the total expected number of days per 100 patients on each arm along with 95% bootstrap CIs. Below each panel is the estimated difference in ICU days per 100 patients (placebo minus remdesivir). Abbreviations: CI, confidence interval; ICU, intensive care unit; NIPPV, noninvasive positive-pressure ventilation.