Abstract
Several studies have revealed either self-reported chemosensory alterations in large groups or objective quantified chemosensory impairments in smaller populations of patients diagnosed with COVID-19. However, due to the great variability in published results regarding COVID-19-induced chemosensory impairments and their follow-up, prognosis for chemosensory functions in patients with such complaints remains unclear. Our objective is to describe the various chemosensory alterations associated with COVID-19 and their prevalence and evolution after infection. A cross-sectional study of 704 healthcare workers with a RT–PCR-confirmed SARS-CoV-2 infection between 2020 February 28 and 2020 June 14 was conducted 3–7 months after onset of symptoms. Data were collected with an online questionnaire. Outcomes included differences in reported chemosensory self-assessment of olfactory, gustatory, and trigeminal functions across time points and Chemosensory Perception Test scores from an easy-to-use at-home self-administered chemosensory test. Among the 704 participants, 593 (84.2%) were women, the mean (SD) age was 42 (12) years, and the questionnaire was answered on average 4.8 (0.8) months after COVID-19. During COVID-19, a decrease in olfactory, gustatory, and trigeminal sensitivities was reported by 81.3%, 81.5%, and 48.0%, respectively. Three to 7 months later, reduced sensitivity was still reported by 52.0%, 41.9%, and 23.3%, respectively. Chemosensory Perception Test scores indicate that 19.5% of participants had objective olfactory impairment. These data suggest a significant proportion of COVID-19 cases have persistent chemosensory impairments at 3–7 months after their infection, but the majority of those who had completely lost their olfactory, gustatory, and trigeminal sensitivities have improved.
Keywords: COVID-19, anosmia, parosmia, long term, taste, trigeminal system
Introduction
Coronavirus disease-2019 (COVID-19) is an ongoing major public health challenge. Olfactory dysfunction (OD) is a specific symptom that may affect approximately 60% of patients suffering from COVID-19 (Spinato et al. 2020; von Bartheld et al. 2020; Whitcroft and Hummel 2020) and is now considered as a stronger indicator of COVID-19 than fever, cough, and shortness of breath (Gerkin et al. 2021).
OD can be quantitative or qualitative. Quantitative OD is defined by a reduction of olfactory sensitivity that can be either a complete (anosmia) or a partial (hyposmia) loss of olfactory function (Hummel et al. 2016). Qualitative OD describes an altered perception of olfactory stimuli: For example, parosmia is defined as the perception of qualitatively altered smells, and phantosmia is defined as the perception of a smell in the absence of an objective odorant (Hummel et al. 2016; Sjölund et al. 2017). Overall, the prevalence of OD in the general population is around 20% (Landis et al. 2004; Yang and Pinto 2016), and all different forms of OD are associated with reduced quality of life (Croy et al. 2014). In addition to OD, COVID-19 also appears to affect other chemosensory modalities, that is, gustation and trigeminal function (Cooper et al. 2020; Parma et al. 2020).
Olfactory and other chemosensory dysfunctions may have detrimental effects. First, affected individuals can expose themselves to harmful substances such as smoke, gas, or spoiled food (Gonzales and Cook 2007; Schiffman 2007). It may trigger dysfunctional nutritional patterns like increased salt and sugar consumption or anorexia (Mattes et al. 1990; Aschenbrenner et al. 2008). Individuals with OD also have higher rates of anxiety and depression (Croy et al. 2014; Kohli et al. 2016). Moreover, a functioning olfactory system may be a necessity in some workplaces, such as healthcare, where staff are required to have the ability to detect and qualify the smell of urine, excrement, infected wounds, or abnormal smells of breath (Kelly 2012).
Investigation of the long-term effects of COVID-19 on chemosensory function is hindered by the recent onset of the pandemic and other challenges: First, many studies on the prevalence of OD during COVID include a relatively small number of participants (Hintschich et al. 2020; Le Bon et al. 2021) or participants with severe forms of COVID-19 (Moein et al. 2020; Speth et al. 2020). Second, many studies on the prevalence of OD during COVID-19 also include participants with an unclear diagnosis of COVID-19 and/or self-diagnosis (Hopkins et al. 2020; Parma et al. 2020). Lastly, although individuals with anosmia can usually evaluate their olfactory function with accuracy (Lötsch and Hummel 2019), this self-assessment is often challenging for individuals with intermediate forms of OD (e.g., hyposmia) (Landis et al. 2003). Finally, studies on persistent post-COVID-19 OD in the past year have used various designs (objective measures [Lechien et al. 2021], semiobjective [Petrocelli et al. 2021], or self-reported [Havervall et al. 2021; Hopkins et al. 2021b] and collected data at varying time intervals after onset of disease. For these reasons, to this date, no consensus has been reached regarding the prevalence of post-COVID-19 OD (Xydakis et al. 2021).
To comprehensively understand long-term olfactory, gustatory, and trigeminal alterations after COVID-19, we analyzed questionnaire responses from a cohort of healthcare workers infected with SARS-CoV-2 during the first wave of the pandemic (2020 February–2020 June). We also developed a Chemosensory Perception Test (CPT), a formal test employing common household odorants and tastants to enable accessible yet accurate self-evaluation of chemosensory functions remotely on a large scale. The CPT is particularly useful when in-person testing is unsafe and testing a large group of participants at distance with mailable tests such as the UPSIT (Doty et al. 1984) is costly. Moreover, distance testing has been reported to accurately monitor disease progression in at-risk populations (Vaira et al. 2020a; Weiss et al. 2020).
Materials and methods
Participants
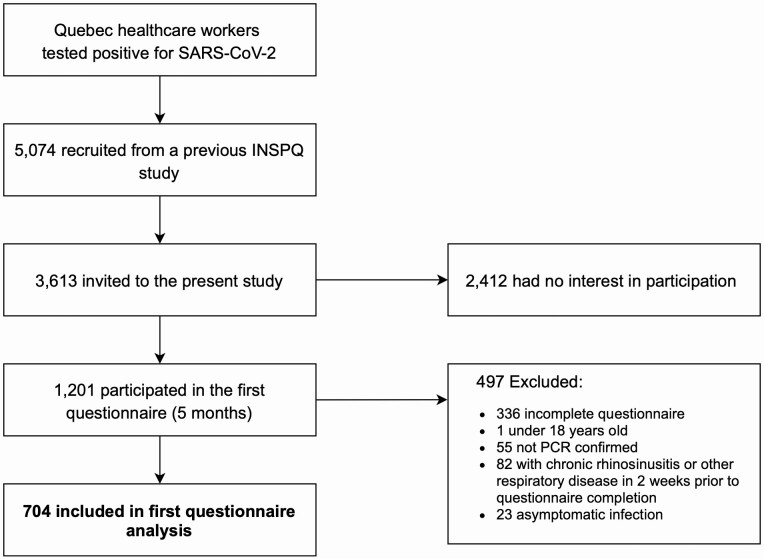
Participants were recruited from a Quebec healthcare worker cohort who have had SARS-CoV-2 infection between 2020 February 28 and 2020 June 14. They were part of a study from the Institut National de Santé Publique du Québec and had agreed to be contacted for other research projects(Carazo et al. 2021). Inclusion criteria were as follows: (i) RT–PCR-confirmed COVID-19, (ii) above 18 years of age, (iii) French or English speakers, (iv) completed the online questionnaire, and (v) did not report of other respiratory diseases (bacterial or viral infection, or/and allergies with rhinorrhea) within 2 weeks prior to questionnaire completion or chronic sinusitis (Fig. 1).
Fig. 1.
Flowchart of participant inclusion/exclusion procedures. Flowchart of the study design. INSPQ, Institut national de santé publique.
This study was reviewed and approved by the research ethics board of the CHU de Québec-Université Laval (MP-20-2021-5228), and all protocols were reviewed by an independent Scientific Review Committee. This study also complies with the Declaration of Helsinki for Medical Research Involving Human Subjects. All participants provided an online informed consent prior to participation. The study received funding from the Fonds de recherche du Québec-Santé. No compensation or incentive was offered for participation. Data were collected from 2020 August 11 to 2020 October 29. Up to 4 attempts were made to reach by email potential participants. At the time of data collection, participants were 3–7 months after the onset of COVID-19 symptoms.
Online questionnaire
All participants were asked to complete an online questionnaire that was adapted from the core questionnaire of the Global Consortium on Chemosensory Research (Parma et al. 2020).
Demographic information.
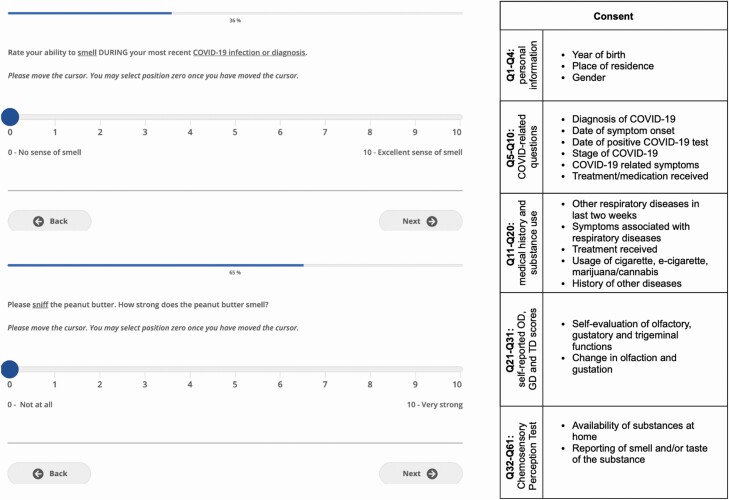
In the first part of the questionnaire, demographic information was collected from all participants. Participants were then instructed to provide medical history and indicate the presence of specific COVID-19 symptoms (Fig. 2).
Fig. 2.
Web-based interface and structure of the online questionnaire. Left, Self-rating of olfaction and reporting of CPT using VAS through the web-based interface, as viewed by the participant. Right, Sections of the online questionnaire. VAS, visual analog scale.
Chemosensory self-assessment.
Participants were asked to self-evaluate and report their olfactory, gustatory, and trigeminal sensitivity using a 10-point visual analog scale (VAS; Fig. 2) for 3 time points: (i) before SARS-CoV-2 infection, (ii) during SARS-CoV-2 infection, and (iii) at questionnaire completion. The specific definition of each chemosensory modality was presented prior to self-evaluation of each chemosensory modality as follows: Olfaction: The following questions relate to your sense of smell (for example, sniffing flowers or soap, or smelling garbage) but not the flavor of food in your mouth; Gustation: The following questions are related to your sense of taste. For example, sweetness, sourness, saltiness, bitterness experienced in the mouth; Trigeminal: The following questions are related to other sensations in your mouth, like burning, cooling, or tingling. For example, chili peppers, mint gum or candy, or carbonation. Furthermore, information on the presence of parosmia or phantosmia following the infection (Landis et al. 2010) and alterations in the 5 tastes (sweet, salty, sour, bitter, umami) was collected.
Chemosensory Perception Test.
Items commonly found in North American households were used to assess participants’ olfactory and gustatory functions, as odor intensity is the best single predictor to classify individuals with normosmia (Parma et al. 2021). Participants had to smell 3 substances (peanut butter, jam/jelly, and coffee) and rate odor intensity on a 10-point VAS (0 = no smell at all; 10 = very strong smell). We obtained olfactory scores by averaging these ratings. Pilot data on a total of 93 participants show these scores to accurately detect OD when compared to the Sniffin’ Sticks (cutoff score: 6/10; sensitivity: 0.765; specificity: 0.895; Supplement 3). Participants were asked to prepare saline and sweet water by dissolving respectively a teaspoon of salt or 3 teaspoons of sugar in a cup (250 mL) of lukewarm water. Then, they were asked to taste saline and sweet water and to rate taste intensities on a 10-point VAS. We obtained gustatory scores by averaging these ratings. An ongoing study is comparing CPT gustatory scores with the Waterless-Empirical Taste Test—Self-Administered (Doty et al. 2021), but too few participants have been recruited to this to establish its accuracy (Supplementary Material 3).
Statistical analyses
A Python script (Python 3.7.5, Python Software Foundation, https://www.python.org) was used to process raw questionnaire data and to calculate the number of participants reporting COVID-19 symptoms, chronic conditions and recent respiratory illnesses. Processed data were analyzed and visualized with SPSS 26.0 (IBM Corp, Armonk, NY), GraphPad Prism 8.3.1 (GraphPad Prism Software, San Diego, CA) and Raincloud plots(Allen et al. 2021).
Parametric (ANOVA) or nonparametric (Friedman) tests were chosen depending on whether normality assumption was fulfilled. To evaluate the effects of COVID-19 on modality (olfactory, gustatory, and trigeminal) and time (prior to, during, and after COVID-19 infection), for gender (women, men), repeated-measures (rm) ANOVA with age as a covariate were computed. To disentangle interactions, separate rmANOVA were carried out for individual modalities and time points with the same factors. Greenhouse–Geisser corrections were used for sphericity and Tukey’s multiple comparisons test were used for post hoc comparisons. Friedman’s test was followed by Dunn’s post hoc test to correct for multiple comparisons. To assess the correlation between self-reported olfactory, gustatory, and trigeminal abilities and results of the CPT, Pearson correlation coefficient or Spearman’s rank correlation coefficient was used. For all statistical tests, alpha was set at 0.05. All results are expressed as mean (SD) unless otherwise specified.
Results
Characteristics of participants
A total of 704 healthcare workers (593 [84.2%] women, mean age of 42.0 [SD: 11.7, range 18 –70] years) were included. The questionnaire was completed on average 4.8 (SD: 0.8, range 3–7) months after symptoms onset. COVID-19 symptoms reported by the 704 participants are listed in Table 1.
Table 1.
COVID-19 symptoms of the 704 participants
| Symptoms at time of SARS-CoV-2 infection | No. (%) |
|---|---|
| Fever | 353 (50.1) |
| Dry cough | 361 (51.7) |
| Cough with mucus | 77 (10.9) |
| Dyspnea | 316 (44.9) |
| Chest tightness | 201 (28.6) |
| Runny nose | 226 (32.1) |
| Sore throat | 330 (46.9) |
| Changes in food flavor | 471 (66.9) |
| Changes in smell | 520 (73.9) |
| Loss of appetite | 323 (45.9) |
| Headache | 518 (73.6) |
| Muscle aches | 444 (63.1) |
| Fatigue | 611 (86.8) |
| Diarrhea | 259 (36.8) |
| Abdominal pain | 102 (14.5) |
| Nausea | 179 (25.4) |
Quantitative disorders
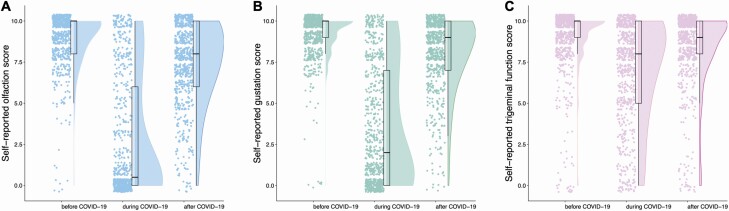
Before COVID-19, average self-reported score was 9.0 (1.6), 9.2 (1.3), and 8.9 (1.9) of 10 for olfaction, gestation, and trigeminal function, respectively. Among participants, 0.9%, 0.7%, and 1.8%, respectively, reported an absence of olfaction, gestation, and trigeminal function (score 0; Fig. 3). During COVID-19, average self-reported score was 2.6 (3.6) for olfaction, 3.4 (3.6) for gustation, and 7.0 (3.0) for trigeminal sensitivity. In the 704 participants, 51.1%, 33.5%, and 5.7% reported absence of olfaction, gustation, and trigeminal function. At time of questionnaire completion, mean scores were 7.4 (2.5), 8.0 (2.2), and 8.5 (2.2) for olfaction, gustation, and trigeminal function, respectively, and the absence of chemical senses was reported, respectively, by 1.4%, 0.7%, and 2.3%. Weak correlations were found between the time since infection and the self-reported olfactory and gustatory scores at questionnaire completion (olfaction: ρ = 0.11; gustation: ρ = 0.14; both P < 0.001; trigeminal ρ = 0.06; P = 0.11).
Fig. 3.
Self-reported scores for the chemosensory modalities before, during, and after COVID-19 infection (n = 704). Raincloud plot representing self-reported scores for olfaction, gustation, and trigeminal function before, during, and after COVID-19. Ratings from individual participants are displayed as dots. Boxplots show the first to third quartiles, horizontal line denotes the median, and whiskers denote 1.5 times interquartile range. Compared with baseline, self-reported scores of olfaction, gustation, and trigeminal function were significantly lower during COVID-19 and have not fully returned to baseline values 5 months after COVID-19.
Compared with the baseline chemosensory functions before COVID-19, 572 (81.3%), 574 (81.5%), and 338 (48.0%) reported lower olfactory, gustatory, and trigeminal sensitivity during COVID-19, respectively. Olfactory and gustatory dysfunction were present in similar proportions (χ 2(2, N = 704) = 0.02, P = 0.891) and were different to trigeminal (olfaction: χ 2(2, N = 704) = 174.81 P < 0.001; gustation: χ 2(2, N = 704) = 174.56, P < 0.001). Three to 7 months after the infection, 366 (52.0%), 295 (41.9%), and 164 (23.3%) reported lower olfactory, gustatory, and trigeminal sensitivity compared to before COVID-19 (Table 2), respectively. These proportions were significantly different between all 3 chemosensory systems (χ 2(2, N = 704) = 123.46, P < 0.001).
Table 2.
Self-reported chemosensory alterations by age group and gender during and 3–7 months following COVID-19 (n = 704)
| During acute COVID-19 | 3–7 months after COVID-19 | ||||||
|---|---|---|---|---|---|---|---|
| Olfaction | Gustation | Trigeminal | Olfaction | Gustation | Trigeminal | ||
| Age | Gender | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) |
| 18–29 | M (N = 11) | 9 (81.8) | 9 (81.8) | 4 (36.4) | 6 (54.5) | 5 (45.5) | 3 (27.3) |
| F (N = 115) | 106 (92.2) | 106 (92.2) | 54 (47.0) | 68 (59.1) | 55 (47.8) | 16 (13.9) | |
| 30–39 | M (N = 26) | 23 (88.5) | 21 (80.8) | 12 (46.2) | 13 (50.0) | 7 (26.9) | 4 (15.4) |
| F (N = 153) | 133 (86.9) | 129 (84.3) | 77 (50.3) | 83 (54.2) | 63 (41.2) | 37 (24.2) | |
| 40–49 | M (N = 33) | 23 (69.7) | 23 (69.7) | 13 (39.4) | 12 (36.4) | 9 (27.3) | 5 (15.2) |
| F (N = 165) | 142 (85.5) | 137 (83.0) | 83 (50.3) | 97 (58.8) | 77 (46.7) | 42 (25.5) | |
| 50–59 | M (N = 28) | 13 (46.4) | 14 (50.0) | 9 (32.1) | 7 (25.0) | 7 (25.0) | 4 (14.3) |
| F (N = 128) | 100 (78.1) | 102 (79.7) | 66 (51.6) | 62 (48.4) | 57 (44.5) | 39 (30.5) | |
| 60+ | M (N = 13) | 5 (38.5) | 6 (46.2) | 5 (38.5) | 2 (15.4) | 1 (7.7) | 3 (23.1) |
| F (N = 32) | 19 (59.4) | 27 (84.4) | 15 (46.9) | 16 (50.0) | 14 (43.8) | 11 (34.4 | |
| Total (N = 704) | 572 (81.3) | 574 (81.5) | 338 (48.0) | 366 (52.0) | 295 (41.9) | 164 (23.2) |
Overall, there were significant effects of modality (F(2,1402) = 42.83, P < 0.001, = 0.058; olfactory < gustatory < trigeminal; all P < 0.001), time (F(2,1402) = 118.47, P < 0.001, = 0.145; during < after < before; all P < 0.001), and gender (F(1,701) = 5.52, P = 0.019, = 0.008; women < men) and significant interactions between these factors (modality × time, modality × time × gender; all P < 0.001) on chemosensory self-evaluation. To disentangle these interactions, we analyzed data separately per chemosensory modality and time points.
Chemosensory modality.
With regards to olfactory function, significant main effects of time (F(2,1402) = 165.07, P < 0.001 = 0.191; during < after < before; all P < 0.001; Fig. 3A), age (F(1,701) = 4.42, P = 0.012, = 0.009), and gender (F(1,701) = 4.42, P = 0.036, = 0.006; women < men) were revealed. In addition, we observed significant interactions of time × age (F(2,1402) = 23.39, P < 0.001, = 0.032) and time × gender (F(2, 1402) = 21.69, P < 0.001, = 0.030).
With regards to gustatory function, we observed significant main effects of time (F(2,1402) = 102.97, P < 0.001, = 0.128; during < after < before; all P < 0.001; Fig. 3B) and gender (F(1, 701) = 9.80, P = 0.002, = 0.014; women < men), but no effect of age. We also observed significant interactions of time × age (F(2, 1402)) = 5.97, P = 0.005, = 0.008) and time × gender (F(2, 1402)) = 20.02, P < 0.001, = 0.028).
With regards to trigeminal function, we observed significant main effects of time (F(2,1402) = 3.91, P = 0.020, = 0.006; during < after < before; all P < 0.001; Fig. 3C), and age (F(1,701) = 5.08, P = 0.025, = 0.007) but no effect of gender. We also identified significant interactions of time × age (F(2, 1402) = 4.70, P = 0.016, = 0.007) and time × gender (F(2, 1402) = 4.50, P = 0.019, = 0.006).
Time point.
With regards to chemosensory function before infection, we observed a significant effect of gender (F(1,701) = 8.52, P = 0.004, = 0.012; men < women), but not of modality, age, or interactions. During COVID-19, we observed a significant effects of modality (F(2, 1402) = 96.714, P < 0.001, = 0.121; olfaction < gustation < trigeminal; all P < .001), gender (F(1, 701) = 21.98, P < 0.001, = 0.030; women < men), and age (F(1, 701) = 4.74, P = 0.030, = 0.007). Furthermore, we found significant interactions modality × age (F(2, 1402) = 24.185, P < 0.001, = 0.033) and modality × gender (F(2, 1402) = 6.76, P =.002, = 0.010). Finally, after infection, we observed a significant effect of modality (F(2, 1402) = 9.91, P < 0.001, = 0.014; olfaction < gustation < trigeminal; all P < 0.015), but not of gender or age, nor any interaction.
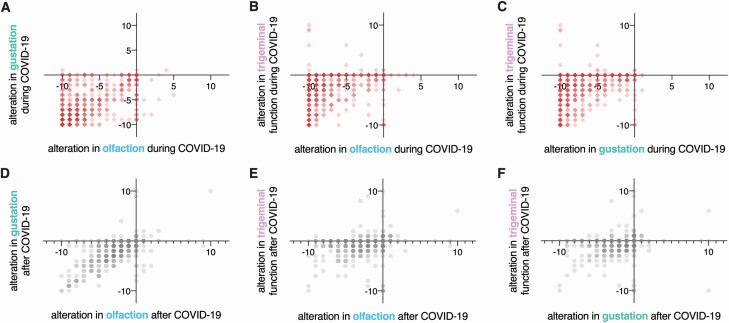
Compared with baseline (before infection), changes in chemosensory function were correlated for all modalities during infection (olfaction–gustation: ρ = 0.69; gustation–trigeminal: ρ = 0.43; olfaction–trigeminal: ρ = 0.33; all P < 0.001, Fig. 4A–C) and after infection (olfaction–gustation: ρ = 0.69; gustation–trigeminal: ρ = 0.40; olfaction–trigeminal: ρ = 0.36; all P < 0.001, Fig. 4D–F).
Fig. 4.
Correlations between alterations in chemosensory modalities (n = 704). Red squares, correlations between alterations in olfaction, gustation, and trigeminal functions during COVID-19. Gray hexagons, correlations between alterations in olfaction, gustation, and trigeminal functions after COVID-19. Darker colors indicate higher occurrence.
Qualitative disorders
Among included participants, 78 (11.1%) reported parosmia, 73 (10.4%) experienced phantosmia, and/or 82 (11.6%) had waxing and waning of olfaction following infection. In addition, 42 (6.0%) claimed that they experienced other forms of OD (hyposmia to specific substances, hyperosmia, parosmia only at high concentrations or slow identification times).
Furthermore, 335 (47.6%) participants reported changes to perception of sweet, 338 (48.0%) salty, 293 (41.6%) sour, 309 (43.9%) bitter, and 281 (39.9%) umami. A total of 275 (39.1%) participants reported alterations in all 5 tastes.
Chemosensory perception test
Among the 704 participants, 137 (19.5%) had a CPT score suggestive of OD. Mean CPT scores were lower for olfaction than gustation (7.84 (1.78) vs 8.42 (2.31); Z = 8.193, P < 0.001). Neither age nor gender had an effect on CPT scores. CPT scores correlated with self-reported chemosensory abilities at testing time (olfaction: ρ = 0.67; gustation: ρ = 0.51; P < 0.001 for both).
Discussion
This study reports chemosensory dysfunction 3 to 7 months following SARS-CoV-2 infection in a large cohort of RT–PCR-confirmed health care workers. In addition to confirming the now well-established detrimental effect of acute COVID-19 on all 3 chemosensory systems (olfactory, gustatory, trigeminal), our major findings are as follows: (i) the detrimental effect of COVID-19 lasts beyond the acute phase after the infection, half of those affected indicated that olfactory function had not returned to the baseline levels 3–7 months later, whereas 20% of infected participants reported scores in a formal test that are consistent with the presentation of hyposmia/anosmia; (ii) approximately 10% of the patients exhibit parosmia and/or phantosmia; and (iii) women are more heavily affected than men.
We observed chemosensory dysfunction in the acute phase of COVID-19, which was most pronounced for olfactory function, but less so for gustatory function and even less for trigeminal function. The proportion of participants describing OD and GD in the acute phase of COVID-19 in this study was comparable to earlier studies (Hajikhani et al. 2020). Although the proportions of participants indicating a decrease in olfaction or gustation were comparable, the olfactory system seems to be more severely impaired. Given the cross-sectional design of the present study, recall bias may have a role to play in the prevalence of OD and GD in similar study populations, but published studies with little to no recall bias also report equivalent prevalence of OD and GD (Andrews et al. 2020; Lechien et al. 2020; Petrocelli et al. 2021). Longitudinal studies are needed to further assess the relationship between OD and GD in COVID-19. Nevertheless, on average 4.8 months after infection and thus well after the acute phase, approximately 50% and 40% of patients reported persistent alterations in olfactory and gustatory function, respectively; these numbers are higher than what has been reported in some studies (Boscolo-Rizzo et al. 2021; Capelli and Gatti 2021; Lechien et al. 2021) and lower than reported by others (Hopkins et al. 2021b). The great variability in these results is due to very different study designs (self-report vs. psychophysical test; prospective vs. cross-sectional) and studied populations (of different ethnicity and under different effects of selection bias), which either influence the measure of OD and GD in study populations or directly impact the baseline prevalence of OD and GD during COVID-19, offsetting all prevalence calculated at further points (Mazzatenta et al. 2020; von Bartheld et al. 2020). For instance, in the study population included in this study, prevalence of OD decreases to 18.9% of participants when measured using the CPT at 4.8 (SD: 0.8) months after infection. The difference in these frequencies could be due to a higher sensitivity of the self-reported alterations compared with the CPT. Participants with milder forms of persistent hyposmia or with higher baseline olfactory sensitivity may have higher scores on the semiobjective CPT yet have not recovered entirely. We found a moderate-to-strong correlation between self-reported olfactory and gustatory changes, which were stronger than with self-reported trigeminal changes. This could be due to similar pathophysiological alterations in the olfactory and gustatory systems and their differences from that of the trigeminal system. Knowing that the general population often mixes up retro-olfaction (perceiving odors from the substances in the mouth traveling posteriorly and rostrally to the olfactory epithelium) with taste, an alternative explanation would be a misunderstanding of this nuance by participants despite the fact that specific definitions for each modality were given (Landis et al. 2005; Malaty and Malaty 2013). The latter hypothesis is more probable since the correlation between gustatory self-report and CPT gustatory scores using strict gustatory stimuli (salt, sugar) is lower than the correlation between olfactory self-report and CPT olfactory scores. When tasting strictly gustatory stimuli in the CPT, participants reflect solely on their sense of taste, without the influence of retronasal sensations. These tests have the potential to be more accurate than simple subjective measures and could simplify large-scale psychophysical chemosensory testing. Others have reported the usefulness of similar self-administered chemosensory tests in the detection and follow-up of COVID-19-induced chemosensory dysfunctions (Vaira et al. 2020c; Petrocelli et al. 2021). Different theories have been proposed to explain the persistence of OD in certain individuals, ranging from olfactory epithelium dysfunction to central nervous system infection (Bilinska and Butowt 2020; Butowt and von Bartheld 2020; Solomon 2021). Since cells of the olfactory epithelium possess the ability to regenerate, the reestablishment of olfactory function is possible in the context of postinfectious OD (Cavazzana et al. 2018), as well as in COVID-19-related OD, where 75–85% of the affected individuals recovered olfactory function within 60 days (Mullol et al. 2020; Lechien et al. 2021). The exact rate of olfactory recovery is still unknown, whereas post-COVID-19 OD prevalence ranges from 11% to 60% at 6 months according to a recent study (Xydakis et al. 2021). In addition to OD and GD, TD has also been reported in patients with COVID-19 (Cooper et al. 2020; Parma et al. 2020).
Persistent chemosensory dysfunctions may be a sign of chronic central nervous system alterations (Gori et al. 2020; Wu et al. 2020), and there is now evidence that SARS-CoV-2 can infect olfactory sensory neurons in humans (Meinhardt et al. 2021; de Melo et al. 2021). Other viruses, such as the Japanese encephalitis virus, Varicella-Zoster virus, measles virus, human immunodeficiency virus, and CoVs, were shown to invade the CNS (Koyuncu et al. 2013). Febrile seizures, loss of consciousness, convulsions, ataxia, status epilepticus, encephalitis, myelitis, neuritis, and extrapyramidal symptoms are among extrapulmonary symptoms that have been described (Bohmwald et al. 2018). However, no evidence of intraparenchymal replication has been found yet. Additional findings include the presence of local immune processes (Saussez et al. 2021) and persistence of viral fragments in the olfactory epithelium (de Melo et al. 2021). Therefore, chronic post-COVID-19 inflammation in the olfactory pathway (epithelium, bulb) with or without direct infection is the most probable pathophysiological explanation of post-COVID-19 OD (Kirschenbaum et al. 2020; Vaira et al. 2020b; Xydakis et al. 2021). The persistence of postinfectious neurological inflammation may contribute to the development or aggravation of chronic neurological diseases such as Parkinson’s disease, multiple sclerosis, or psychiatric outcomes (Morris 1985; Johnson-Lussenburg and Zheng 1987; Fazzini et al. 1992; Murray et al. 1992; Stewart et al. 1992; Cristallo et al. 1997; Arbour et al. 2000; Koyuncu et al. 2013; Cohen et al. 2020; Taquet et al. 2021). These patients should be followed up to document the development of neurological sequalae.
Moreover, approximately 10% reported parosmia and/or phantosmia following SARS-CoV-2 infection. These qualitative smell disorders usually involve unpleasant olfactory sensations (rotten eggs, sewage, smoke). While the exact patho-mechanism of parosmia and phantosmia are still to be elucidated, parosmia is probably linked to altered peripheral input/central processing of olfactory stimuli (Iannilli et al. 2019). Importantly, patients with postviral OD and parosmia exhibit better recovery rates following olfactory training than those without parosmia (Liu et al. 2021). Follow-ups will determine to what extent parosmia predicts a better outcome.
Women’s chemical senses were more affected than men during and after COVID-19 infection. Women typically have better scores in olfactory testing than men at baseline (Wang et al. 2019). However, in line with our results, studies have revealed that women exhibit a higher prevalence and a longer persistence of postviral OD (Liu et al. 2016; Sorokowski et al. 2019). Gender differences could be explained by a multitude of neuroendocrine, social, and cognitive factors (Sorokowski et al. 2019). We also found that older individuals have lower olfactory and gustatory sensitivities, especially during the acute phase of COVID-19.
Currently, there is no approved therapy specifically for COVID-19-induced OD, although experts agree that olfactory training could be prescribed for COVID-induced OD as it has a significant effect on olfactory function according to studies on other viral infections (Damm et al. 2014; Sorokowska et al. 2017; Doty 2019; Huart et al. 2021). Additionally, oral steroids, intranasal steroids and/or omega-3 supplements may be prescribed on an individual basis (Hopkins et al. 2021a). Most importantly, long-term follow-up of these patients will be necessary to assess other signs of neurological damage or spontaneous recovery, as recoveries can be possible after a year in other postviral OD (Lee et al. 2014).
Limitations
Given the cross-sectional design of the study, a recall bias is possible for all self-reported peri-SARS-CoV-2 infection values before or during the SARS-CoV-2 infection due to the 3- to 7-month gap. This study did not control for potential confounding factors such as race and level of education. Finally, the CPT requires further validation for its gustatory and trigeminal components, and it relies on substances found in participants’ homes, which may lead to variation in test results due to the differences in the brand, quality, or expiration date of substances and consequently, their ability to trigger equal sensorineural responses.
Conclusions
Nearly two thirds of SARS-CoV-2 infected patients had chemosensory impairments during their infection, and despite improvements, impairments persist in half of them 3–7 months after COVID-19. Quantitative and qualitative OD as well as persisting gustatory and trigeminal deficits were common in the cohort presented in this study. Given the frequency of these problems and the possible neurological underpinnings of these observations, it will be critical to understand the underlying mechanisms of these chemosensory dysfunctions, their evolution, and possible therapeutic options.
Supplementary Material
Acknowledgments
We thank Josiane Rivard for preparing the online questionnaire; Cécilia Tremblay, Émilie Aubry-Lafontaine, and Frédérique Roy-Côté for data collection and the validation of the Chemosensory Perception Test; and all study participants and frontline healthcare workers facing the COVID-19 pandemic.
Funding
This work was supported by Fonds de recherche du Québec – Santé (chercheur boursier junior 2 #283144 to J.F.). N.B. and J.F. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. F.G.L. is the recipient of a tier-2 Canada research Chair. All authors declare no conflict of interest.
Conflict of Interest
FGL is the recipient of a tier-2 Canada research Chair. All authors declare no conflict of interest.
References
- Allen M, Poggiali D, Whitaker K, Marshall T, van Langen J, Kievit R. 2021. Raincloud plots: a multi-platform tool for robust data visualization. Wellcome Open Res. 4(63). doi: 10.12688/wellcomeopenres.15191.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PJ, Pendolino AL, Ottaviano G, Scarpa B, Grant J, Gaudioso P, Bordin A, Marchese-Ragona R, Leoni D, Cattelan A, et al. . 2020. Olfactory and taste dysfunction among mild-to-moderate symptomatic COVID-19 positive health care workers: an international survey. Laryngosc Investig Otolaryngol. 5(6):1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour N, Day R, Newcombe J, Talbot PJ. 2000. Neuroinvasion by human respiratory coronaviruses. J Virol. 74(19):8913–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner K, Hummel C, Teszmer K, Krone F, Ishimaru T, Seo HS, Hummel T. 2008. The influence of olfactory loss on dietary behaviors. Laryngoscope. 118(1):135–144. [DOI] [PubMed] [Google Scholar]
- Bilinska K, Butowt R. 2020. Anosmia in COVID-19: a bumpy road to establishing a cellular mechanism. ACS Chem Neurosci. 11(15):2152–2155. doi: 10.1021/acschemneuro.0c00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. 2018. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 12:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo-Rizzo P, Guida F, Polesel J, Marcuzzo AV, Antonucci P, Capriotti V, Sacchet E, Cragnolini F, D’Alessandro A, Zanelli E, et al. . 2021. Self-reported smell and taste recovery in coronavirus disease 2019 patients: a one-year prospective study. Eur Arch Oto-rhino-laryngol. 1–6. doi: 10.1007/s00405-021-06839-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R, von Bartheld CS. 2020. Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelli M, Gatti P. 2021. Anosmia in the first coronavirus disease 2019 outbreak in Europe: functional recovery after eight months. J Laryngol Otol. 135(3):224–228. 10.1017/S0022215121000670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo SLD, Villeneuve J, Martin R, Deshaies P, Denis G, Adib G, Tissot F, Dionne M, De Serres G. 2021. Characterization and evolution of infection control practices among SARS-CoV-2 infected healthcare workers of acute care hospitals and long-term care facilities in Quebec, Canada, Spring 2020. Infect Contr Hospit Epidemiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzana A, Larsson M, Münch M, Hähner A, Hummel T. 2018. Postinfectious olfactory loss: a retrospective study on 791 patients. Laryngoscope. 128(1):10–15. [DOI] [PubMed] [Google Scholar]
- Cohen ME, Eichel R, Steiner-Birmanns B, Janah A, Ioshpa M, Bar-Shalom R, Paul JJ, Gaber H, Skrahina V, Bornstein NM, Yahalom G. 2020. A case of probable Parkinson’s disease after SARS-CoV-2 infection. Lancet Neurol. 19(10):804–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KW, Brann DH, Farruggia MC, Bhutani S, Pellegrino R, Tsukahara T, Weinreb C, Joseph PV, Larson ED, Parma V, Albers MW, Barlow LA, Datta SR, Di Pizio A. 2020. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 107(2):219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristallo A, Gambaro F, Biamonti G, Ferrante P, Battaglia M, Cereda PM. 1997. Human coronavirus polyadenylated RNA sequences in cerebrospinal fluid from multiple sclerosis patients. New Microbiol. 20(2):105–114. [PubMed] [Google Scholar]
- Croy I, Nordin S, Hummel T. 2014. Olfactory disorders and quality of life—an updated review. Chem Senses. 39(3):185–194. [DOI] [PubMed] [Google Scholar]
- Damm M, Pikart LK, Reimann H, Burkert S, Göktas Ö, Haxel B, Frey S, Charalampakis I, Beule A, Renner B, Hummel T, Hüttenbrink KB. 2014. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope. 124(4):826–831. [DOI] [PubMed] [Google Scholar]
- de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, Verillaud B, Aparicio C, Wagner S, Gheusi G, Kergoat L, Kornobis E, Donati F, Cokelaer T, Hervochon R, Madec Y, Roze E, Salmon D, Bourhy H, Lecuit M, Lledo PM. 2021. COVID-19–related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 13(596):eabf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. Treatments for smell and taste disorders: a critical review. 2019. Handb Clin Neurol. 164:455–479. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Kimmelman CP, Dann MS. 1984. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 94(2 Pt 1):176–178. [DOI] [PubMed] [Google Scholar]
- Doty RL, Wylie C, Potter M. 2021. Validation of the Waterless Empirical Taste Test (WETT®). Behav Res Methods. 53(2):864–873. [DOI] [PubMed] [Google Scholar]
- Fazzini E, Fleming J, Fahn S. 1992. Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson’s disease. Mov Disord. 7(2):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerkin R, Ohla K, Veldhuizen M, Joseph P, Kelly C, Bakke A, Steele K, Farruggia M, Pellegrino R, Pepino M, et al. . 2021. Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chem Senses. 46. doi: 10.1093/chemse/bjaa081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales GM, Cook MJ. 2007. Chapter 13—disorders of smell and taste. In: Schapira AHV, Byrne E, DiMauro S, et al. , editors. Neurology and clinical neuroscience. Philadelphia (PA): Mosby. p. 171–177. [Google Scholar]
- Gori A, Leone F, Loffredo L, Cinicola BL, Brindisi G, De Castro G, Spalice A, Duse M, Zicari AM. 2020. COVID-19-related anosmia: the olfactory pathway hypothesis and early intervention. Front Neurol. 11:956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajikhani B, Calcagno T, Nasiri MJ, Jamshidi P, Dadashi M, Goudarzi M, Eshraghi AA, Mirsaeidi M. 2020. Olfactory and gustatory dysfunction in COVID-19 patients: a meta-analysis study. Physiol Rep. 8(18):e14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havervall S, Rosell A, Phillipson M, Mangsbo SM, Nilsson P, Hober S, Thålin C. 2021. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 325(19):2015–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintschich CA, Wenzel JJ, Hummel T, Hankir MK, Kühnel T, Vielsmeier V, Bohr C. 2020. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. Int Forum Allergy Rhinol. 10(9):1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C, Alanin M, Philpott C, Harries P, Whitcroft K, Qureishi A, Anari S, Ramakrishnan Y, Sama A, Davies E, Stew B, Gane S, Carrie S, Hathorn I, Bhalla R, Kelly C, Hill N, Boak D, Nirmal Kumar B. 2021a. Management of new onset loss of sense of smell during the COVID-19 pandemic—BRS Consensus Guidelines. Clin Otolaryngol. 46(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C, Surda P, Vaira LA, Lechien JR, Safarian M, Saussez S, Kumar N. 2021b. Six month follow-up of self-reported loss of smell during the COVID-19 pandemic. Rhinology. 59(1):26–31. [DOI] [PubMed] [Google Scholar]
- Hopkins C, Surda P, Whitehead E, Kumar BN. 2020. Early recovery following new onset anosmia during the COVID-19 pandemic—an observational cohort study. J Otolaryngol Head Neck Surg. 49(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huart C, Philpott CM, Altundag A, Fjaeldstad AW, Frasnelli J, Gane S, Hsieh JW, Holbrook EH, Konstantinidis I, Landis BN, et al. . 2021. Systemic corticosteroids in coronavirus disease 2019 (COVID-19)-related smell dysfunction: an international view. Int Forum Allergy Rhinol. 11(7):1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, Damm M, Frasnelli J, Gudziol H, Gupta N, et al. . 2016. Position paper on olfactory dysfunction. Rhinology. 56(1):1–30. [DOI] [PubMed] [Google Scholar]
- Iannilli E, Leopold DA, Hornung DE, Hummel T. 2019. Advances in understanding parosmia: an fMRI study. ORL J Otorhinolaryngol Relat Spec. 81(4):185–192. [DOI] [PubMed] [Google Scholar]
- Johnson-Lussenburg CM, Zheng Q. 1987. Coronavirus and multiple sclerosis: results of a case/control longitudinal serological study. Adv Exp Med Biol. 218:421–429. [DOI] [PubMed] [Google Scholar]
- Kelly M. 2012. Scent of a patient: an underestimated role in clinical practice? Br J Gen Pract. 62(600):378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum D, Imbach LL, Ulrich S, Rushing EJ, Keller E, Reimann RR, Frauenknecht KBM, Lichtblau M, Witt M, Hummel T, Steiger P, Aguzzi A, Frontzek K. 2020. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet. 396(10245):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli P, Soler ZM, Nguyen SA, Muus JS, Schlosser RJ. 2016. The association between olfaction and depression: a systematic review. Chem Senses. 41(6):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncu OO, Hogue IB, Enquist LW. 2013. Virus infections in the nervous system. Cell Host Microbe. 13(4):379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis BN, Frasnelli J, Croy I, Hummel T. 2010. Evaluating the clinical usefulness of structured questions in parosmia assessment. Laryngoscope. 120(8):1707–1713. [DOI] [PubMed] [Google Scholar]
- Landis BN, Frasnelli J, Reden J, Lacroix JS, Hummel T. 2005. Differences between orthonasal and retronasal olfactory functions in patients with loss of the sense of smell. Arch Otolaryngol Head Neck Surg. 131(11):977–981. [DOI] [PubMed] [Google Scholar]
- Landis BN, Hummel T, Hugentobler M, Giger R, Lacroix JS. 2003. Ratings of overall olfactory function. Chem Senses. 28(8):691–694. [DOI] [PubMed] [Google Scholar]
- Landis BN, Konnerth CG, Hummel T. 2004. A study on the frequency of olfactory dysfunction. Laryngoscope. 114(10):1764–1769. [DOI] [PubMed] [Google Scholar]
- Le Bon SD, Pisarski N, Verbeke J, Prunier L, Cavelier G, Thill MP, Rodriguez A, Dequanter D, Lechien JR, Le Bon O, Hummel T, Horoi M. 2021. Psychophysical evaluation of chemosensory functions 5 weeks after olfactory loss due to COVID-19: a prospective cohort study on 72 patients. Eur Arch Otorhinolaryngol. 278:101–108. doi: 10.1007/s00405-020-06267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien JR, Chiesa-Estomba CM, Beckers E, Mustin V, Ducarme M, Journe F, Marchant A, Jouffe L, Barillari MR, Cammaroto G, Circiu MP, Hans S, Saussez S. 2021. Prevalence and 6-month recovery of olfactory dysfunction: a multicentre study of 1363 COVID-19 patients. J Intern Med. 290(2):451–461. [DOI] [PubMed] [Google Scholar]
- Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, et al. . 2020. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 277:2251–2261. doi: 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Lee W, Wee J, Kim JW. 2014. Prognosis of postviral olfactory loss: follow-up study for longer than one year. Am J Rhinol Allergy. 28(5):419–422. doi: 10.2500/ajra.2014.28.4102. [DOI] [PubMed] [Google Scholar]
- Liu J, Pinto JM, Yang L, Li L, Sun J, Miao X, Li K, Chen G, Wei Y. 2016. Gender difference in Chinese adults with post-viral olfactory disorder: a hospital-based study. Acta Otolaryngol. 136(9):976–981. [DOI] [PubMed] [Google Scholar]
- Liu DT, Sabha M, Damm M, Philpott C, Oleszkiewicz A, Hähner A, Hummel T. 2021. Parosmia is associated with relevant olfactory recovery after olfactory training. Laryngoscope. 131(3):618–623. [DOI] [PubMed] [Google Scholar]
- Lötsch J, Hummel T. 2019. Clinical usefulness of self-rated olfactory performance-a data science-based assessment of 6000 patients. Chem Senses. 44(6):357–364. [DOI] [PubMed] [Google Scholar]
- Malaty J, Malaty IA. 2013. Smell and taste disorders in primary care. Am Fam Phys. 88(12):852–859. [PubMed] [Google Scholar]
- Mattes R, Cowart B, Schiavo M, Arnold C, Garrison B, Kare M, Lowry L. 1990. Dietary evaluation of patients with smell and/or taste disorders. Am J Clin Nutr. 51:233–240. [DOI] [PubMed] [Google Scholar]
- Mazzatenta A, Neri G, D’Ardes D, De Luca C, Marinari S, Porreca E, Cipollone F, Vecchiet J, Falcicchia C, Panichi V, Origlia N, Di Giulio C. 2020. Smell and taste in severe Covid-19: self-reported vs. testing. Front Med. 7:589409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brünink S, Greuel S, et al. . 2021. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 24:168–175. doi: 10.1038/s41593-020-00758-5 [DOI] [PubMed] [Google Scholar]
- Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. 2020. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 10(8):944–950. doi: 10.1002/alr.22587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA. 1985. Clinical viral infections and multiple sclerosis. Lancet. 2(8449):273. [DOI] [PubMed] [Google Scholar]
- Mullol J, Alobid I, Mariño-Sánchez F, Izquierdo-Domínguez A, Marin C, Klimek L, Wang DY, Liu Z. 2020. The loss of smell and taste in the COVID-19 outbreak: a tale of many countries. Curr Allergy Asthma Rep. 20(10):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RS, Brown B, Brian D, Cabirac GF. 1992. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann Neurol. 31(5):525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V, Hannum ME, O’Leary M, Pellegrino R, Rawson NE, Reed DR, Dalton PH. 2021. SCENTinel 1.0: development of a rapid test to screen for smell loss. Chem Senses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, Cooper KW, Bouysset C, Pirastu N, Dibattista M, et al. . 2020. More than smell. COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 45(7):609–622. doi: 10.1093/chemse/bjaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocelli M, Cutrupi S, Salzano G, Maglitto F, Salzano FA, Lechien JR, Saussez S, Boscolo-Rizzo P, De Riu G, Vaira LA. 2021. Six-month smell and taste recovery rates in coronavirus disease 2019 patients: a prospective psychophysical study. J Laryngol Otol. 135(5):436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saussez S, Sharma S, Thiriad A, Olislagers V, Vu Duc I, Le Bon SD, Khalife M, Hans S, De Riu G, Hopkins C, Lechien JR, Vaira LA, Marchant A. 2021. Predictive factors of smell recovery in a clinical series of 288 coronavirus disease 2019 patients with olfactory dysfunction. Eur J Neurol. 00:1–10. doi: 10.1111/ene.14994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS. 2007. Smell and taste. In: Birren JE, editor. Encyclopedia of gerontology. 2nd ed. New York (NY): Elsevier, 515–525. [Google Scholar]
- Sjölund S, Larsson M, Olofsson JK, Seubert J, Laukka EJ. 2017. Phantom smells: prevalence and correlates in a population-based sample of older adults. Chem Senses. 42(4):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T. 2021. Neurological infection with SARS-CoV-2—the story so far. Nat Rev Neurol. 17(2):65–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokowska A, Drechsler E, Karwowski M, Hummel T. 2017. Effects of olfactory training: a meta-analysis. Rhinology. 55(1):17–26. [DOI] [PubMed] [Google Scholar]
- Sorokowski P, Karwowski M, Misiak M, Marczak MK, Dziekan M, Hummel T, Sorokowska A. 2019. Sex differences in human olfaction: a meta-analysis. 10(242). doi: 10.3389/fpsyg.2019.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth MM, Singer-Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR. 2020. Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg. 163(1):114–120. doi: 10.1177/0194599820929185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C, Boscolo-Rizzo P. 2020. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 323(20):2089–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JN, Mounir S, Talbot PJ. 1992. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 191(1):502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 2021. 6-Month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 8(5):P416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M, Ferrari M, Gagliardini L, Pipolo C, Deiana G, et al. . 2020a. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 42(7):1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira LA, Hopkins C, Sandison A, Manca A, Machouchas N, Turilli D, Lechien JR, Barillari MR, Salzano G, Cossu A, Saussez S, De Riu G. 2020b. Olfactory epithelium histopathological findings in long-term coronavirus disease 2019 related anosmia. J Laryngol Otol. 134(12):1123–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira LA, Salzano G, Petrocelli M, Deiana G, Salzano FA, De Riu G. 2020c. Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck. 42(7):1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS, Hagen MM, Butowt R. 2020. Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. ACS Chem Neurosci. 11(19):2944–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang C, Xia X, Yang Y, Zhou C. 2019. Effect of gender on odor identification at different life stages: a meta-analysis. Rhinology. 57(5):322–330. [DOI] [PubMed] [Google Scholar]
- Weiss JJ, Attuquayefio T, White EB, Geng B, Handoko R, Herz RS, White TL, Iwasaki A, Grubaugh ND, Datta R, et al. . 2020. 456. Implementing an at-home smell test for early assessment of COVID-19 in high-risk healthcare workers. Open Forum Infectious Dis. 7(Suppl 1):S295–S296. [Google Scholar]
- Whitcroft KL, Hummel T. 2020. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. 323(24):2512–2514. [DOI] [PubMed] [Google Scholar]
- Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. 2020. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 87:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xydakis MS, Albers MW, Holbrook EH, Lyon DM, Shih RY, Frasnelli JA, Pagenstecher A, Kupke A, Enquist LW, Perlman S. 2021. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 20(9):753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Pinto JM. 2016. The epidemiology of olfactory disorders. Curr Otorhinolaryngol Rep. 4(2):130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.