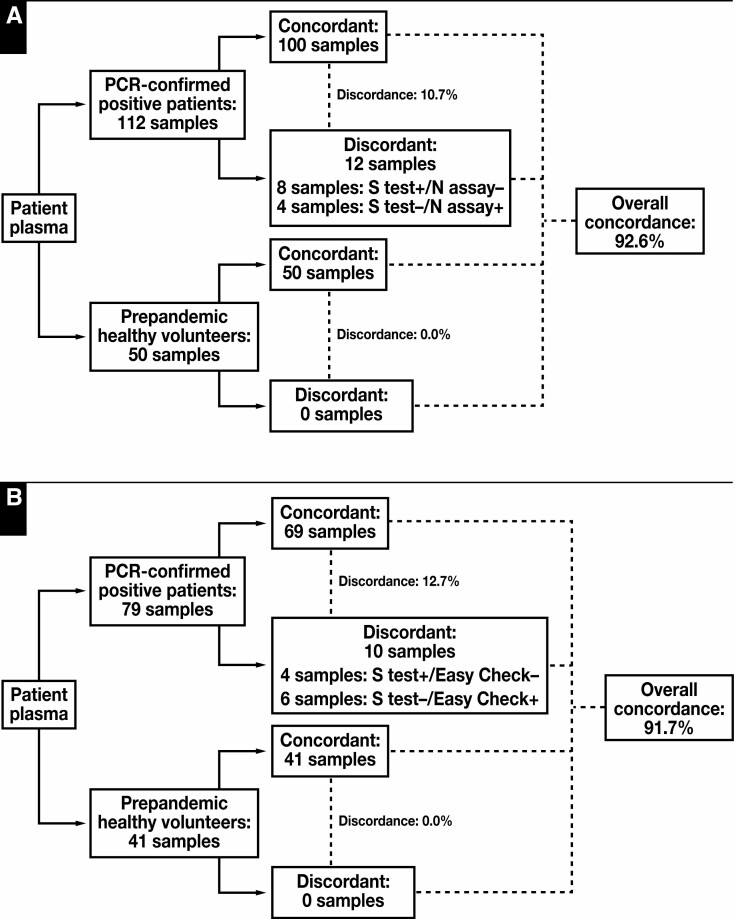
Figure 4.
Method comparison of the Elecsys anti–SARS-CoV-2 S-Ab semiquantitative assay vs the Elecsys anti–SARS-CoV-2 nucleocapsid antibody (N-Ab) qualitative assay (A) and the Easy Check lateral flow device (B). The overall concordance between the spike protein (S-Ab) assay and the N-Ab assay or the Easy Check device are greater than 90%. Discordant cases are comprised of both positive and negative S-Ab assay results, which likely reflect differences in test principle and/or the specific analytes assayed between the 3 methods. PCR, polymerase chain reaction.