Abstract
Background and Objectives:
Near-infrared fluorescence (NIRF) of the Firefly® system has become a useful and widespread technique for the visualization and detection of tumors, sentinel lymphnodes, and vascular/anatomical structures.
Methods:
Between February 1, 2017 to September 30, 2019, a total of 25 patients affected by benign and malignant pathologies underwent robotic surgery by the use of organ transillumination with the concomitant Firefly®. We analyzed the pre-operative patients' characteristics (age and body mass index [BMI], previous abdominal surgeries and systemic disease); pre-operative diagnosis, surgical procedure and approach (multiport or single site), transilluminated organ, surgical outcomes (operating time, incidence of intraoperative complications, and incidence of conversion to other surgery); and postoperative outcome. The surgical procedures included: four bladder endometriosis nodules resections, one pelvic lymphadenectomy with ureterolysis, and 23 hysterectomies.
Results:
The average operating time was 283.3 (+/- 76.9) minutes, there were no intra-operative complications or laparotomic conversions. The average recovery days were 5.9. There have been three grade 2 post-surgical complications, following the Memorial Sloan Kettering Cancer Center Surgical Secondary Events System classification. The combination of NIRF and transillumination allows a clear view of the anatomical landmarks and the resection margins.
Conclusions:
It’s likely that improvement in the anatomical detail could confer a greater surgical safety with lower percentage of intra and post-surgical complications and sparing of safe tissue. To evaluate the validity of these techniques in a larger number of patients and compare these new surgical procedures with standard ones, further studies are needed.
Keywords: Firefly system, Organ Transillumination, Anatomical landmark
INTRODUCTION
Robotic surgery may contribute to overcoming technical limitations of standard laparoscopy thanks to its high-definition, three-dimensional vision provided by the optical system, the seven degrees of freedom of its instruments, the tremor-filtering mechanism, and the stability of the optical platform, thereby facilitating the performance of complex minimally invasive procedures.1,2
Firefly® has been available since 2011 as an option to the da Vinci system. The need for a better intra-surgical view has significantly increased the interest toward fluorescence imaging. Near-infrared fluorescence has become a useful and increasingly widespread technique for the visualization and detection of tumors, sentinel lymphnodes, and vascular/anatomical structures. Da Vinci utilizes the near-infrared fluorescence (NIRF) technique to identify the tracer (indocyanine green, [ICG]) in the blood/lymphatic system, which, once provided intravenously or via lymphatic system, binds to plasma proteins and gets access to the vascular system.3,4
ICG can be injected either intravenously to assess the vascularization of specific tissues or “in situ” directly into various organs to identify diseased parenchyma or to assess the lymphatic pathways.
The use of NIRF/ICG improves the identification of key anatomical landmarks and pathological structures for oncological and nononcological procedures.1,2
NIR light has a wavelength of 700 – 900 nm and it is invisible to the naked eye. This technology provides real time imaging, which represents a fundamental guidance during the surgical procedure.
NIR fluorescence can penetrate deeply (for a few millimeters) in blood and soft tissues and it is absorbed more by biomolecules (e.g., hemoglobin or lipids) than by water, thus allowing the identification of deeper anatomical structures.
In addition to indocyanine green, Firefly® can be utilized along with other devices which allow organ transillumination; indeed NIRF light even captures green colored images of selected organs, like the ureter or the bladder.5–10
In this study, we report surgical and clinical outcomes of a retrospective cohort of 25 patients who underwent robotic surgery with the use of organ transillumination and concomitant FireFly® system. To our knowledge, this is the first study published on this topic.
MATERIALS and METHODS
This is a retrospective case review of 25 patients who underwent robotic surgery because of endometrial or cervical cancer, uterine fibromatosis, and bladder endometriosis.
The surgical procedures, including hysterectomy, radical hysterectomy, pelvic lymphadenectomy, bladder endometriosis resection, were performed by surgeons M.R., S.B., A.V., and D.S., using da Vinci™ Surgical Robot (Intuitive Surgical Inc., Sunnyvale, CA, USA). The study is exempt from Institutional Review Board approval.
We analyzed the pre-operative patients' characteristics (age and body mass index [BMI], parity, previous abdominal surgeries, and systemic disease), pre-operative diagnosis, surgical procedure and surgical approach (multiport or single–site), transilluminated organ, surgical outcomes (operating time, incidence of intra-operative complications, and incidence of conversion to other surgery); and postoperative outcomes such as readiness to discharge according to the Post-Anesthetic Discharge Scoring System (PADSS), the drop of hemoglobin, and the incidence of post-operative complications according to Memorial Sloan Kettering Cancer Center’s Surgical Secondary Events (SSE) System. (Table 1).
Table 1.
Surgical Procedures
| Four arms robotics procedures | 14 |
| Single site robotic procedures | 11 |
| Hysterectomy | 12 |
| Hysterectomy and sampling of pelvic lymph nodes | 1 |
| Hysterectomy and pelvic lymphadenectomy | 6 |
| Hysterectomy, pelvic, and lombo-aortic lymphadenectomy | 1 |
| Hysterectomy, pelvic lymphadenectomy, and transurethral resection of bladder | 1 |
| Bladder resections of endometriotic nodules | 4 |
SURGICAL TECHNIQUES
Hysterectomy with Vaginal Transillumination and Firefly® System
This study included patients who underwent hysterectomy for different reasons: fibromatosis, complex atypical hyperplasia, endometrial carcinoma with or without pelvic, and/or paraaortic lymphadenectomy with multiport or single port techniques.11,12
In all hysterectomy procedures, a uterine manipulator called SecuFix (Richard Wolf) was used to make colpotomy easier, improve the visualization of the selected area, and more vaginal tissue. SecuFix provides transillumination of vaginal fornixes, as it features a annular light at the level of the disposable cap. (Figure 1)
Figure 1.

A) Vaginal Fornix With Colpotransillumination B) Vaginal Fornix With Colpotransillumination And FireFly® System Near-infrared Fluorescence Light.
The simultaneous activation of Firefly® allows the surgeon to better identify the margins of the manipulator’s valve and, thus, the colpotomy’s borders, allowing them to adjust the surgical cut and spare viable tissue wherever possible.
Hysterectomy, Left Pelvic Lymphadenectomy, and Transurethral Resection of the Bladder with Ureters Transillumination and Firefly® System
Firefly® was also employed in 1 case of bladder carcinoma. The patient recruited in this study, showed a bladder lesion of 1.5 cm on computed tomography and a left pelvic lymph node of increased dimensions that was in close anatomical proximity to the homolateral ureter. After the staging procedure, the patient underwent robotic hysterectomy and left pelvic lymphadenectomy.1,2 Before the procedure, a Transilluminating Ureteric Stent (Rocket URIGLOW) was placed via cystoscopy.
Through the use of this device, the operator has been able to safely perform the lymphadenectomy with the Firefly® technique while observing the ureteral course; the latter was lightened in green and was well distinguished (Figure 2), thus reducing the probability of an accidental organ injury.
Figure 2.
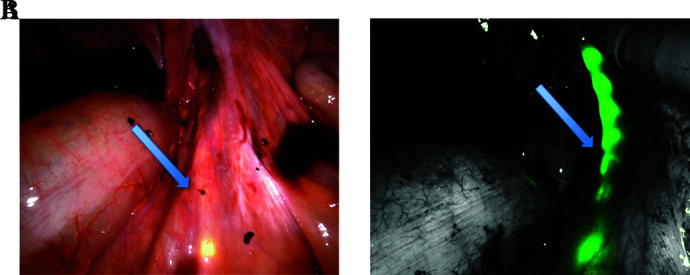
A) Right Ureter With Transilluminating Stent B) Right Ureter With Transilluminating Stent And FireFly® System Near-infrared Fluorescence Light.
At the end of the gynecological procedure, the urologist removed the bladder lesion via transurethral resection of the bladder, which was then defined histologically as an invasive non-papillary carcinoma G3.
Bladder Endometriosis with Bladder Trans-Illumination and Firefly® System
Firefly® has been used on patients undergoing bladder resection due to endometriosis.13 The cystoscopic transillumination of the bladder and the simultaneous activation of the Firefly® system has allowed surgeons to precisely identify bladder lesions. The bladder, filled up with physiologic solution and then lightened with cystoscope light, appears as green colored. The organ damage, on the other hand, can be recognized as a dark area, not lightened up, whose margins appear well defined and easily removable.
STATISTICAL ANALYSIS
The results of parametric data are expressed as a median. Excel software (Microsoft Inc.) was used for all statistical analyses. Confidence intervals were set at 95%.
RESULTS
From February 1, 2017 to September 30, 2019, four bladder endometriosis nodules resections, one pelvic lymphadenectomy with ureterolysis, and 23 hysterectomies (25 patients total) were performed with organ transillumination (bladder, ureter, vaginal fornix) and the Firefly® System. All operations were performed following the standard surgical techniques.
Twelve patients underwent hysterectomy for benign pathology (11 because of fibromatosis and one because of complex atypical hyperplasia). Eight patients underwent hysterectomy for malignant pathology, 7 of whom had pelvic lymphadenectomy and one with pelvic and paraortic lymphadenectomy. (Table 1)
Median age was 52.2 (+/- 13) years, BMI was 24.9 (+/- 3.3).
Most of the patients recruited for this study had already undergone surgical procedures (8 previous laparotomies, 6 previous laparoscopies, 5 previous caesarean sections)
The average operating time was 283.3 (+/-76.9) mins, There were no intra-operative complications or laparotomic conversions.
Patients were considered ready to be discharged when PADSS was > 9. This score was reached medially at day 3.6 of hospitalization (4.3 for patients with oncological disease and 3.4 for women affected by endometriosis or fibromatosis).
There were three grade 2 postsurgical complications (Table 2), following the Memorial Sloan Kettering Cancer Center’s SSE System: one abdominal wall hematoma and two pneumonitis. One patient affected by endometrial carcinoma developed a parietal hematoma at the site of the left lateral trocar insertion, to be then discharged on the eighth day; she lost 4.9 grams/deciliter (g/dl) of hemoglobin and underwent intravenous iron therapy using ferric carboxymaltose.
Table 2.
Surgical and Post-Operative Outcomes
| Operating time (Mins) | 283 ± 76.9 |
| Intra-operative complication | 0 |
| Conversion to laparoscopy or laparotomy | 0 |
| Hemoglobin drop (g/dl) | 1.7 ± 1.2 |
| Postoperative complication | 3 |
| Readiness to discharge (days) | 3.6 ± 2.3 |
Two patients suffered from pneumonitis during the recovery, stayed in the hospital for 10 days, and were treated with intravenous antibiotic therapy.
Complete surgical and postoperative outcomes are summarized in Table 2.
DISCUSSION
The proper identification of key anatomical structures is mandatory to achieve successful outcomes, particularly in complex surgical procedures.
Transillumination has been thoroughly described in literature as a clinical tool to improve anatomical visualization, particularly to detect diverticula and bladder endometriosis.5–10,14,15
The application of NIRF technology has been proposed in many specialties; its major application in gynecology is the identification of the sentinel lymph node during the surgical staging of the endometrial or cervical carcinoma. The advantage in identifying the sentinel lymph node relies on the fact that lymphadenectomy has a purely staging, non-therapeutic purpose in the treatment of malignant uterine pathologies, therefore avoiding systemic lymphadenectomy (especially in older, debilitated, obese patients with multiple comorbidities), and substantially reduces intra and post-surgical complications, as well as the operative time.16–23
In our clinical practice, Firefly® and indocyanine green have been used on a daily basis in surgeries for many years, especially for the uterine oncologic staging, as previously outlined. It is indeed evident that the combination of NIRF and transillumination has greatly improved surgical performances, allowing a clear view of the anatomical landmarks and the resection margins. It is important to recall that a better operative view may improve the surgical dissection, while sparing healthy tissue and, most importantly, by reducing the percentage of complications related to accidental organ injury.
In our study, there were no intra-operative complications or laparotomic conversions. This highlights the fact that an improvement in anatomical detail can confer greater surgical safety, which can also be interpreted in a lower percentage of intra and post-surgical complications while also saving healthy tissue.
However, to confirm that the association between transillumination and NIRF increases accuracy in identifying anatomical structures, while improving intra- and postoperative outcomes, further studies are necessary.
Further case-control studies will be required in the future to verify whether there are actual advantages in terms of anatomical structures identification, intra- and postoperative outcomes, using the organ transillumination associated with NIRF compared to transillumination alone.
Footnotes
Disclosure: none.
Conflict of interests: none.
Funding sources: none.
Informed consent: Dr. Maurizio Rosati declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
Contributor Information
Maurizio Rosati, Department of Gynecology and Obstetrics, Santo Spirito Hospital, Pescara, Italy..
Silvia Bramante, Department of Gynecology and Obstetrics, Santo Spirito Hospital, Pescara, Italy..
Alessandro Vigone, Department of Gynecology and Obstetrics, University Hospital Maggiore della Carita, Novara, Italy..
Martina Gerbino, Department of Gynecology and Obstetrics, University Hospital Maggiore della Carita, Novara, Italy..
Fiorella Conti, Department of Gynecology and Obstetrics, Santo Spirito Hospital, Pescara, Italy..
Serena Mauri, Department of Obstetrics and Gynecology, University of Chieti, Chieti, Italy..
Daniela Surico, Department of Translational Medicine, University of Eastern Piedmont, Novara, Italy..
References:
- 1.Cho JE, Nezhat FR. Robotics and gynecologic oncology: review of the literature. J Minim Invasive Gynecol. 2009; Nov-Dec 16(6):669–681. [DOI] [PubMed] [Google Scholar]
- 2.Nezhat F. Minimally invasive surgery in gynecologic oncology: laparoscopy versus robotics. Gynecol Oncol. 2008;111(2 Suppl):S29–S32. [DOI] [PubMed] [Google Scholar]
- 3.Reinhart MB, Huntington CR, Blair LJ, Heniford BT, Augenstein VA. Indocyanine green: historical context, current applications, and future considerations. Surg Innov. 2016; Apr;23(2):166–175. [DOI] [PubMed] [Google Scholar]
- 4.Spinoglio G, Bertani E, Borin S, Piccioli A, Petz W. Green indocyanine fluorescence in robotic abdominal surgery. Updates Surg. 2018. Sep;70(3):375–379. [DOI] [PubMed] [Google Scholar]
- 5.Dikici S, Aldemir Dikici B, Eser H, et al. Development of a 2-dof uterine manipulator with LED illumination system as a new transvaginal uterus amputation device for gynecological surgeries. Minim Invasive Ther Allied Technol. 2018; Jun27(3):177–185. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez MM, Pedroso JD, Volker KW, Howard DL, McCarus SD. The McCarus-Volker ForniSee®: a novel trans-illuminating colpotomy device and uterine manipulator for use in conventional and robotic-assisted laparoscopic hysterectomy. Surg Technol Int. 2017;30(30):191–196. [PubMed] [Google Scholar]
- 7.Phipps JH, Tyrrell NJ. Transilluminating ureteric stents for preventing operative ureteric damage. Br J Obstet Gynaecol. 1992;99(1):81. [DOI] [PubMed] [Google Scholar]
- 8.Putz A, Skrøppa S. Total laparoscopic hysterectomy using a new uterine manipulator. J Minim Invasive Gynecol. 2015;22(6S):S151. [DOI] [PubMed] [Google Scholar]
- 9.Rebouças RB, Monteiro RC, Souza T., et al. Laparoscopic bladder diverticulectomy assisted by cystoscopic transillumination. Int Braz J Urol. 2014;40(2):281–282. [DOI] [PubMed] [Google Scholar]
- 10.Seracchioli R, Mannini D, Colombo FM, Vianello F, Reggiani A, Venturoli S. Cystoscopy-assisted laparoscopic resection of extramucosal bladder endometriosis. J Endourol. 2002;16(9):663–666. [DOI] [PubMed] [Google Scholar]
- 11.Nezhat C, Lavie O, Lemyre M, Unal E, Nezhat CH, Nezhat F. Robot-assisted laparoscopic surgery in gynecology: scientific dream or reality? Fertil Steril. 2009;91(6):2620–2622. [DOI] [PubMed] [Google Scholar]
- 12.Nezhat C, Lavie O, Lemyre M, Gemer O, Bhagan L, Nezhat C. Laparoscopic hysterectomy with and without a robot: Stanford experience. JSLS. 2009;13(2):125–128. [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Perisic D, Peresic D, Samadi D, Nezhat F. Robotic-assisted laparoscopic partial bladder resection for the treatment of infiltrating endometriosis. J Minim Invasive Gynecol. 2008;15(6):745–748. [DOI] [PubMed] [Google Scholar]
- 14.Thwaini A, McLeod A, Nambirajan T. Laparoscopic bladder diverticulectomy. J Laparoendosc Adv Surg Tech A. 2008;18(6):849–851. [DOI] [PubMed] [Google Scholar]
- 15.Yu Y, Guan S, Shen C, Yang B. Sequential transuretral enucleation of the prostate and laparoscopic bladder diverticulectomy. Minim Invasive Ther Allied Technol. 2016;25(4):222–224. [DOI] [PubMed] [Google Scholar]
- 16.Cacciamani GE, Shakir A, Tafuri A, et al. Best practices in near-infrared fluorescence imaging with indocyanine green (NIRF/ICG)-guided robotic urologic surgery: a systematic review-based expert consensus. World J Urol. 2020;38(4):883–896. [DOI] [PubMed] [Google Scholar]
- 17.Emerson J, Robison K. Evaluation of sentinel lymph nodes in vulvar, endometrial and cervical cancers. WJOG. 2016;5(1):78–86. [Google Scholar]
- 18.Lin H, Ding Z, Kota VG, Zhang X, Zhou J. Sentinel lymph node mapping in endometrial cancer: a systematic review and meta-analysis. Oncotarget. 2017;(28):46601–46610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang SW, Ou JJ, Wong HP. The use of indocyanine green imaging technique in patient with hepatocellular carcinoma. Transl Gastroenterol Hepatol. 2018;3:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton AD1, Predina JD1, Nie S, Low PS3, Singhal S. Intraoperative fluorescence imaging in thoracic surgery. J Surg Oncol. 2018;118(2):344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangano A, Masrur MA, Bustos R, Chen LL, Fernandes E, Giulianotti PC. Near-Infrared Indocyan near-Infrared Indocyanine Green Enhanced Fluorescence and Minimally Invasive Colorectal Surgery: Review of the Literature. Surg Technol Int. 2018;33:77–83. [PubMed] [Google Scholar]
- 22.van der Pas MH, van Dongen GA, Cailler F, Pelegrin A, Meijerink WJ. Sentinel node procedure of the sigmoid using indocyanine green: feasibility study in a goat model. Surg Endosc. 2010. 2010;24(9):2182–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veccia A, Antonelli A, Hampton LJ, et al. Near-infrared fluorescence imaging with indocyanine green in robot-assisted partial nephrectomy: pooled analysis of comparative studies. Eur Urol Focus. 2020;6(3):505–512. [DOI] [PubMed] [Google Scholar]


